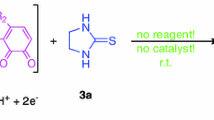
Abstract
Electrosynthesis of 4-iodo-substituted pyrazoles has been accomplished by iodination of the corresponding precursors on a Pt-anode in aqueous solutions of KI under conditions of the diaphragm galvanostatic electrolysis. Efficiency of the process depends on the donor-acceptor properties of substituents and their positions in the pyrazole ring. Thus, iodination of pyrazole, 3,5-dimethylpyrazole, 3-nitropyrazole, 1-methylpyrazole, 1,3-dimethylpyrazole, pyrazole-3(5)-carboxylic acid or methyl esters furnished 4-iodo derivatives in 57, 86, 2, 5, 35, 30, and 40% yields, respectively.
Similar content being viewed by others
References
S. Manfredini, P. G. Baraldi, R. Bazzanini, Nucleosides, Nucleotides, Nucleic Acids, 2000, 19, 705.
D. R. Sliskovic, B. D. Roth, M. W. Wilson, J. Med. Chem., 1990, 33, 31.
B. R. Tolf, R. Dahlbom, H. Theorell, A. Akeson, Acta Chem. Scand., B, 1982, 36, 101.
I. I. Grandberg, D. K. Bareeva, A.s. SSSR No. 320.493; RZhKhim. [Abstr. J. Chem.], 1972, 14N 235 (in Russian).
S. Manfredini, P. G. Baraldi, J. Chem. Soc., Chem. Commun., 1994, 5, 583.
S. Manfredini, P. G. Baraldi, R. Bazzanini, Bioorg. Med. Chem. Lett., 1996, 6, 1279.
J. Elguero, G. Jaramilo, Synthesis, 1997, 563.
V. Collot, P. Dallemagnet, P. P. Bovy, Tetrahedron, 1999, 59, 6917.
B. V. Lyalin, V. A. Petrosyan, B. I. Ugrak, Elektrokhim., 2008, 44, 1418 [Russ. J. Electrochem. (Engl. Transl.), 2008, 44, 1320].
B. V. Lyalin, V. A. Petrosyan, B. I. Ugrak, Izv. Akad. Nauk, Ser. Khim., 2009, 291 [Russ. Chem. Bull., Int. Ed., 2009, 58, 291].
B. V. Lyalin, V. A. Petrosyan, B. I. Ugrak, Elektrokhim., 2010, 46, 131 [Russ. J. Electrochem. (Engl. Transl.), 2010, 46, 123].
R. Hüttel, O. Schäfer, P. Jochim, Justus Liebigs Ann. Chem., 1955, 593, 200.
G. T. Morgan, J. Ackerman, J. Chem. Soc., 1923, 123, 1308.
S. F. Vasilevskii, M. S. Shvartsberg, Izv. Akad. Nauk SSSR, Ser. Khim., 1980, 1071 [Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1980, 29, 778].
I. Nishiguchi, O. Kambe, K. Itoh, Synth. Lett., 2000, 89.
M. S. Pevzner, Adv. Heterocycl. Chem., 1999, 75, 1.
A. J. Gordon, R. A. Ford, The Chemist’s Companion, Wiley, New York—London—Sidney—Toronto, 1972.
B. V. Nekrasov, Uchebnik obshchei khimii [Text-book of General Chemistry], 4th ed., Khimiya, Moscow, 1981, 566 pp. (in Russian).
J. D. Vaughan, D. G. Lambert, V. L. Vaughan, J. Am. Chem. Soc., 1964, 86, 2857.
J. D. Vaughan, G. L. Jewett, J. Am. Chem. Soc., 1967, 89, 6218.
J. L. Huppatz, L. John, Austral. J. Chem., 1983, 38, 135.
J. W. Janssen, H. J. Koeners, C. L. Habraken, J. Org. Chem., 1973, 38, 1777.
Yu. A. Manaev, M. A. Andreeva, V. P. Perevalov, B. I. Stepanov, Zh. Obshch. Khim., 1982, 52, 2592 [J. Gen. Chem.(Engl. Transl.), 1982, 52, 2191].
S. Owen, J. Chem. Soc., 1947, 1033.
R. Miethen, R. Randow, R. Listemann, J. Fuer. Praktische Chem. (Leipzig), 1989, 331, 799.
H. A. Stefani, C. M. P. Pereira, R. B. Almeida, Tetrahedron Lett., 2005, 40, 6833.
K. Moriyama, T. Suzuki, K. Negishi, J. Med. Chem., 2008, 51, 159.
C. G. Wayne, M. Eberle, Heterocycles, 2007, 71, 1967.
I. M. Kolthoff, R. Belcher, Volumetric Analysis, V. III, New York, 1957.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1512–1518, August, 2010.
Rights and permissions
About this article
Cite this article
Lyalin, B.V., Petrosyan, V.A. & Ugrak, B.I. Electrosynthesis of 4-iodopyrazole and its derivatives. Russ Chem Bull 59, 1549–1555 (2010). https://doi.org/10.1007/s11172-010-0277-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0277-y