Abstract
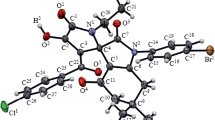
6-Aroyl-7-arylindolo[3,4-jk]phenanthridin-5(4H)-ones (2a–i) were synthesized by heating 3-(2-aryl-2-oxoethylidene)-2,3-dihydroindol-2-ones (1a–i) in DMF. Compounds 2a–i are formed via the dimerization of two molecules of unsaturated ketones 1a–i proceeding as the [2+4] cycloaddition through the formation of intermediate spiro adducts. The further Pfitzinger rearrangement, decarboxylation, and heteroaromatization afford compounds 2a–i. The structures of the reaction products were established by spectroscopic methods and X-ray diffraction.
Similar content being viewed by others
References
A. S. El-Ahl Abdel, H. Afeefy, M. A. Metwally, J. Chem. Res. Miniprint, 1994, 1, 201.
K. S. Joshi, R Jain, Heterocycles, 1990, 31, 473.
A. Dandia, M. Upreti, B. Rani, U. C. Pant, I. J. Gupta, J. Fluorine Chem., 1998, 91, 171.
D. Du Puis, H. G. Lindwall, J. Am. Chem. Soc., 1934, 56, 471.
P. H. Zrike, H. G. Lindwall, J. Am. Chem. Soc., 1935, 57, 207.
R. S. Belen’kaya, E. I. Boreko, M. N. Zemtsova, M. I. Kalinina, M. M. Timofeeva, P. L. Trakhtenberg, V. M. Chelnov, A. E. Lipkin, V. I. Votyakov, Khim.-farm. Zh., 1981, 15, No. 3, 29 [Pharm. Chem. J., 1981, 15, No. 3, 171 (Engl.Transl.)].
A. C. Coda, G. Desimoni, P. P. Righetti, G. Tacconi, E. Dradi, Gazz. Chim. Ital., 1983, 113, 191.
A. B. Serov, V. G. Kartsev, Yu. A. Aleksandrov, Abstrs Intern. Conf. “Chemistry of Nitrogen Containing Heterocycles” (September 30–October 3, 2003), Kharkov, Ukraine, 2003, p. 258.
C. G. Richards, D. E. Thurston, Tetrahedron, 1983, 39, 1817.
G. Tacconi, A. C. Piccolini, P. P. Righetti, E. Selva, G. Desimoni, J. Chem. Soc., Perkin Trans. 1, 1976, 1248.
P. Bamfield, A. W. Jonson, A. F. Katner, J. Chem. Soc. (C), 1966, 1028.
A. Kubo, T. Nakai, T. Nozoye, A. Itai, Y. IItaka, Heterocycles, 1978, 9, 1051.
A. Itai, Y IItaka, A. Kubo, Acta Crystallogr., Sect. B, 1978, 34, 3775.
H. Günter, NMR-Spectroscopie, Georg Thieme Verlag, Stuttgart, 1973.
Yu. V. Zefirov, Kristallografiya, 1997, 42, 936 [Crystallogr. Repts., 1997, 42 (Engl. Transl.)].
H. B. Burgi, J. D. Dunitz, Structure Correlation, 2, VCH, Weinheim, 1994, p. 741.
N. S. Zefirov, V. A. Palyulin, E. E. Dashevskaya, J. Phys. Org. Chem., 1990, 3, 147.
H. G. Lindwall, J. S. Maclennan, J. Am. Chem. Soc., 1932, 54, 4739.
G. Tacconi, P. Iadarola, F. Marinone, P. P. Righetti, G. Desimoni, Tetrahedron, 1975, 31, 1179.
S. Hanessian, Tetrahedron Lett., 1967, 1549.
G. M. Sheldrick, SHELXTL PLUS. PC Version. A System of Computer Programs for the Determination of Crystal Structure from X-ray Diffraction Data, Rev. 5.1, 1998.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 609–618, March, 2008.
Rights and permissions
About this article
Cite this article
Paponov, B.V., Shishkin, O.V., Shishkina, S.V. et al. Dimerization of 3-(2-aryl-2-oxoethylidene)oxindoles. Russ Chem Bull 57, 622–631 (2008). https://doi.org/10.1007/s11172-008-0098-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-008-0098-4