Abstract
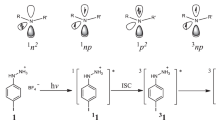
The molecular and crystal structures of 4-amino-2,6-diazido-3,5-dichloropyridine and 6-amino-2,4-diazido-1,3,5-triazine, as well as the paramagnetic photolysis products of their crystals at 77 K, were studied using X-ray diffraction analysis and ESR spectroscopy. Triplet nitrenes generated during the photolysis of diazidopyridine form triplet—triplet nitrene pairs, whose ESR spectrum corresponds to the quintet spin state. The high-spin state (S = 2) results from the exchange interaction between two triplet molecules with the zero-field splitting parameters |D| = 1.0280 cm−1 and |E| = 0.0038 cm−1 and the γ angle between two C—N nitrene bonds equal to 133°. This angle is close to an angle of 136.2° between the C-N bonds of two adjacent molecules in the crystal structure. No formation of the triplet—triplet nitrene pairs is observed during the photolysis of crystalline diazidotriazine, whose molecules lie in the parallel planes.
Similar content being viewed by others
References
Nitrenes, Ed. W. Lwowski, Wiley, New York, 1970.
Azides and Nitrenes, Reactivity and Utility, Ed. E. F. V. Scriven, Academic Press, New York, 1984.
E. F. V. Scriven, K. Turnbull, Chem. Rev., 1988, 88, 297.
G. B. Schuster, M. S. Platz, Adv. Photochem., 1992, 17, 69.
J. Backes, Methoden der organischen Chemie, Houben-Weyl, Georg Thieme Verlag, Stuttgart, 1992, E16C, 67.
R. M. Moriarty, M. Rahman, G. J. King, J. Am. Chem. Soc., 1966, 88, 842.
L. Mahe, A. Izuoka, T. Sugawara, J. Am. Chem. Soc., 1992, 114, 7904.
A. Sasaki, L. Mahe, A. Izuoka, T. Sugawara, Bull. Chem. Soc. Jpn, 1998, 71, 1259.
M. Kawano, T. Takayama, H. Uekusa, Y. Ohashi, Y. Ozawa, K. Matsubara, H. Imabayashi, M. Mitsumi, K. Toriumi, Chem. Lett., 2003, 922.
T. Takayama, M. Kawano, H. Uekusa, Y. Ohashi, T. Sugawara, Helv. Chim. Acta, 2003, 86, 1352.
S. V. Chapyshev, Izv. Akad. Nauk, Ser. Khim., 2006, 1536 [Russ. Chem. Bull., Int. Ed., 2006, 55, 1539].
S. V. Chapyshev, P. M. Lahti, J. Phys. Org. Chem., 2006, 19, 637.
E. Ya. Misochko, A.V. Akimov, V. F. Lavitskii, S. V. Chapyshev, Izv. Akad. Nauk, Ser. Khim., 2007, 2284 [Russ. Chem. Bull., Int. Ed., 2007, 56, No. 12].
S. V. Chapyshev, M. S. Platz, Mendeleev Commun., 2001, 56.
Yu. A. Azev, I. P. Loginova, O. L. Guselnikova, S. V. Shorshnev, N. A. Klyuev, V. L. Rusinov, O. N. Chupakhin, Mendeleev Commun., 1994, 24.
G. M. Sheldrick, SHELXTL v. 6.14 (08/06/2000), Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin, USA.
S. Stoll, A. Schweiger, J. Magn. Reson., 2006, 178, 42.
L. Parkanyi, G. Besenyi, J. Mol. Struct., 2004, 69, 97.
S. I. Kuzina, D. V. Korchagin, G. V. Shilov, S. V. Chapyshev, A. I. Mikhailov, and S. M. Aldoshin, Dokl. Akad. Nauk, 2008, 418, 341 [Dokl. Phys. Chem., 2008 (Engl. Transl.)].
E. Kessenich, T. M. Klapotke, J. Knitzek, H. Noth, A. Schulz, Eur. J. Inorg. Chem., 1998, 2013.
K. Gibson, S. Tragl, H.-J. Meyer, Z. Anorg. Allg. Chem., 2005, 631, 1751.
M.-H. V. Huynh, M. A. Hiskey, E. L. Hartline, D. P. Monyoya, R. Gilardi, Angew. Chem., Int. Ed., 2004, 43, 4924.
M.-H. V. Huynh, M. A. Hiskey, D. E. Chavez, D. L. Naud, R. Gilardi, J. Am. Chem. Soc., 2005, 127, 12537.
S. V. Chapyshev, H. Tomioka, Bull. Chem. Soc. Jpn, 2003, 76, 2075.
S. V. Chapyshev, Mendeleev Commun., 2002, 168.
T. Nakai, K. Sato, D. Shiomi, T. Takui, K. Itoh, M. Kozaki, K. Okada, Mol. Cryst. Liq. Cryst., 1999, 334, 157.
S. V. Chapyshev, R. Walton, P. R. Serwinski, P. M. Lahti, J. Phys. Chem. A, 2004, 108, 6643.
S. V. Chapyshev, Izv. Akad. Nauk, Ser. Khim., 2006, 1085 [Russ. Chem. Bull., Int. Ed., 2006, 55, 1126].
S. V. Chapyshev, Mendeleev Commun., 2003, 53.
K. Itoh, Pure Appl. Chem., 1978, 50, 1251.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 513–520, March, 2008.
Rights and permissions
About this article
Cite this article
Chapyshev, S.V., Lavitskii, V.F., Akimov, A.V. et al. Photochemical generation of triplet—triplet nitrene pairs in aromatic diazide crystals. Russ Chem Bull 57, 524–531 (2008). https://doi.org/10.1007/s11172-008-0081-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-008-0081-0