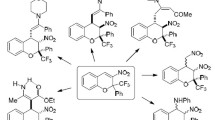
Abstract
Heating of 3-nitro-2-trifluoromethyl-and 3-nitro-2-trichloromethyl-2H-chromenes with indole, N-methylindole, and N-methylpyrrole under solvent-free conditions led with high stereoselectivity and in good yields to cis-trans-3-nitro-2-trifluoromethyl-or trans-cis-3-nitro-2-trichloromethylchromanes substituted by the indol-3-yl (pyrrol-2-yl) fragment in position 4.
Similar content being viewed by others
References
G. P. Ellis, Chromenes, Chromanones, and Chromones, in The Chemistry of Heterocyclic Compounds, Ed. G. P. Ellis, Wiley, New York, 1977, 31.
W. S. Bowers, T. Ohta, J. S. Cleere, and P. A. Marsella, Science, 1976, 193, 542.
R. Bergmann and R. Gericke, J. Med. Chem., 1990, 33, 492.
G. Burrell, F. Cassidy, J. M. Evans, D. Lightowler, and G. Stemp, J. Med. Chem., 1990, 33, 3023.
R. Gericke, J. Harting, I. Lues, and C. Schittenhelm, J. Med. Chem., 1991, 34, 3074.
T. Hiyama, Organofluorine Compounds. Chemistry and Application, Springer Verlag, Berlin, 2000.
V. Yu. Korotaev, I. B. Kutyashev, and V. Ya. Sosnovskikh, Heteroatom Chem., 2005, 16, 492.
V. Yu. Korotaev, V. Ya. Sosnovskikh, I. B. Kutyashev, and M. I. Kodess, Lett. Org. Chem., 2005, 2, 616.
V. Yu. Korotaev, V. Ya. Sosnovskikh, I. B. Kutyashev, and M. I. Kodess, Izv. Akad. Nauk, Ser. Khim., 2006, 309 [Russ. Chem. Bull., Int. Ed., 2006, 55, 317 (Engl. Transl.)].
V. Yu. Korotaev, V. Ya. Sosnovskikh, I. B. Kutyashev, and M. I. Kodess, Izv. Akad. Nauk, Ser. Khim., 2006, 1945 [Russ. Chem. Bull., Int. Ed., 2006, 55, 2020 (Engl. Transl.)].
V. Yu. Korotaev, I. B. Kutyashev, V. Ya. Sosnovskikh, and M. I. Kodess, Mendeleev Commun., 2007, 17, 52.
R. J. Sundberg, The Chemistry of Indoles, Academic Press, New York, 1996.
M. Chakrabarty, R. Basak, N. Ghosh, and Y. Harigaya, Tetrahedron, 2004, 60, 1941.
G. Bartoli, M. Bosco, S. Giuli, A. Giuliani, L. Lucarelli, E. Marcantoni, L. Sambri, and E. Torregiani, J. Org. Chem., 2005, 70, 1941.
S. Iwata, Y. Ishiguro, M. Utsugi, K. Mitsuhashi, and K. Tanaka, Bull. Chem. Soc. Jpn, 1993, 66, 2432.
P. K. Singh, A. Bisai, and V. K. Singh, Tetrahedron Lett., 2007, 48, 1127.
C. Lin, J. Hsu, M. N. V. Sastry, H. Fang, Z. Tu, J.-T. Liu, and Y. Ching-Fa, Tetrahedron, 2005, 61, 11751.
K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025.
N. Ono, N. Banshou, S. Ito, T. Murashima, and T. Ogawa, J. Heterocycl. Chem., 1997, 34, 1243.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1985–1990, October, 2007.
Rights and permissions
About this article
Cite this article
Korotaev, V.Y., Sosnovskikh, V.Y. & Kutyashev, I.B. Reactions of 3-nitro-2-trihalomethyl-2H-chromenes with indole, N-methylindole, and N-methylpyrrole. Stereoselective synthesis of 4-azolyl-3-nitro-2-trihalomethylchromanes. Russ Chem Bull 56, 2054–2059 (2007). https://doi.org/10.1007/s11172-007-0321-8
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-007-0321-8