Abstract
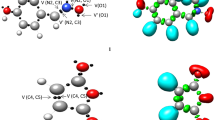
N-Acylalkylation of neutral and anionic N-nucleophiles with α-halocarbonyl compounds was investigated by quantum chemical methods in terms of the density functional theory and by experimental methods for 2,3-dihydroimidazo[2,1-b]quinazolin-1(10)H-5-one, its N-anion, and simpler model structures. High reactivity of these reagents is determined primarily by stabilization of transition states (TS) by bridge bonds involving halogen or nitrogen atoms rather than by conjugation, as has been commonly accepted. Bridged TS are formed by both the substitution mechanism S N 2 and the addition-elimination mechanism. α-Haloalkyl-substituted zwitterions, which are potential intermediates of stepwise N-acylalkylation of neutral N-nucleophiles, do not exist in the isolated state, but they are rather efficiently stabilized upon solvation. These zwitterions, as well as analogous O-anions generated from anionic N-nucleophiles, can serve as intermediates of N-acylalkylation, as was demonstrated by localization of the corresponding TS.
Similar content being viewed by others
References
A. W. Erian, S. M. Sherif, and H. M. Gaber, Molecules, 2003, 8, 793; (b) I. K. Moiseev, M. N. Zemtsova, and N. V. Makarova, Khim. Geterotsikl. Soedin., 1994, 867 [Chem. Heterocycl. Compd., 1994, 30, 745 (Engl. Transl.)].
J. B. Conant and W. R. Kirner, J. Am. Chem. Soc., 1924, 46, 232.
J. B. Conant and R. E. Hussey, J. Am. Chem. Soc., 1925, 47, 476.
J. B. Conant, W. R. Kirner, and R. E. Hussey, J. Am. Chem. Soc., 1925, 47, 488.
R. G. Pearson, S. H. Langer, F. V. Forrest, and W. J. McGuire, J. Am. Chem. Soc., 1952, 74, 5130.
A. J. Sisti and S. Lowell, Can. J. Chem., 1964, 42, 1896.
J. W. Thorpe and J. Warkentin, Can. J. Chem., 1973, 51, 927.
D. M. Kalendra and B. R. Sickles, J. Org. Chem., 2003, 68, 1594.
F. G. Bordwell and W. T. Brannen, J. Am. Chem. Soc., 1964, 86, 4645.
F. Carrion and M. J. S. Dewar, J. Am. Chem. Soc., 1984, 106, 3531.
D. J. McLennan and A. Pross, J. Chem. Soc., Perkin Trans. 2, 1984, 981.
R. D. Bach, B. A. Coddens, and G. J. Wolber, J. Org. Chem., 1986, 51, 1030.
D. Kost and K. Aviram, J. Am. Chem. Soc., 1986, 108, 2006.
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry, Structure and Mechanisms, 4th ed., Kluwer Press, New York-Boston, 2000, A, 302.
T. I. Yousaf and E. S. Lewis, J. Am. Chem. Soc., 1987, 109, 6137.
V. Gineityte, J. Mol. Struct. (Theochem.), 2003, 663, 47.
S. Shaik, J. Am. Chem. Soc., 1983, 105, 4359.
P. D. Bartlett and E. N. Trachtenberg, J. Am. Chem. Soc., 1958, 80, 5808.
J. March, Advanced Organic Chemistry, Reactions, Mechanisms and Structure, Wiley Intersci. Publ., New York, 1985.
T. H. Lowry and K. S. Richardson, Mechanism and Theory in Organic Chemistry, Harper and Row, New York, 1987, 704; 707; 709.
J. W. Baker, Trans. Faraday Soc., 1941, 37, 632.
H. J. Koh, K. L. Han, H. W. Lee, and I. Lee, J. Org. Chem., 2000, 65, 4706.
I. Lee, H. W. Lee, and Y.-K. Yu, Bull. Korean Chem. Soc., 2003, 24, 993.
K. S. Lee, K. K. Adhikary, H. W. Lee, B.-S. Lee, and I. Lee, Org. Biomol. Chem., 2003, 1, 1989.
Organic Syntheses. An Annual Publication of Satisfactory Methods for the Preparation of Organic Chemicals, Ed. L. S. Hegedus, Wiley, Hoboken (New Jersey), 2003, 79, 228.
M. Masaki, K. Fukui, and M. Ohta, J. Org. Chem., 1967, 32, 3564.
T. I. Temnikova and E. N. Kropacheva, Zh. Obshch. Khim., 1949, 19, 1917 [J. Gen. Chem. USSR, 1949, 19 (Engl. Transl.)].
C. L. Stevens, W. Malik, and R. Pratt, J. Am. Chem. Soc., 1950, 72, 4758.
C. L. Stevens and E. Farkas, J. Am. Chem. Soc., 1957, 79, 3448.
D. T. Mowry, Chem. Rev., 1948, 42, 189.
M. Yasuda, T. Ohata, I. Shibata, A. Baba, and H. Matsuda, J. Chem. Soc., Perkin Trans. 1, 1993, 859.
K. Ya. Burstein and A. N. Isaev, J. Mol. Struct. (Theochem.), 1985, 133(26), 263; (b) A. N. Isaev, Izv. Akad. Nauk, Ser. Khim., 1994, 227 [Russ. Chem. Bull., 1994, 43, 206 (Engl. Transl.)].
I. H. Williams, J. Am. Chem. Soc., 1987, 109, 6299.
R. McGrindle and A. J. McAlees, J. Chem. Soc., Chem. Commun., 1983, 61.
F. L. Weisenborn and J. S. P. Schwarz, US Pat. 3360560; http://v3.espacenet.com.
L. V. Saloutina, M. I. Kodess, and A. Ya. Zapevalov, Izv. Akad. Nauk, Ser. Khim., 1994, 2177 [Russ. Chem. Bull., 1994, 43, 2057 (Engl. Transl.)].
M. L. M. Schilling, H. D. Roth, and W. C. Herndon, J. Am. Chem. Soc., 1980, 102, 4271.
H. Diebler and R. N. F. Thorneley, J. Am. Chem. Soc., 1973, 95, 896.
W. P. Jenks, Acc. Chem. Res., 1976, 9, 425.
P. E. Fanta, in The Chemistry of Heterocyclic Compounds, Part 1, Heterocyclic Compounds with Three-and Four-membered Rings, Ed. A. Weissberger, Interscience, New York-London-Sydney, 1964, 548.
G. A. Olah, P. W. Westerman, G. Melby, and Y. K. Mo, J. Am. Chem. Soc., 1974, 96, 3565.
G. A. Olah, Halonium Ions, Wiley-Interscience, New York, 1975.
G. A. Olah, G. K. S. Prakash, R. E. Williams, I. D. Field, and K. Wade, Hypercarbon Chemistry, Wiley-Interscience, New York-Chichester-Brisbane-Toronto-Singapore, 1987.
J. M. Bollinger, J. M. Brinich, and G. A. Olah, J. Am. Chem. Soc., 1970, 92, 4025.
D. Kilemet, Z. Mihalic, I. Novak, and H. Vancik, J. Org. Chem., 1999, 64, 4931.
S. Wolfe, D. J. Mitchell, and H. B. Schlegel, Can. J. Chem., 1982, 60, 1291.
R. M. Minyaev and E. A. Lepin, Mendeleev Commun., 1997, 189.
The Chemistry of Functional Groups. Suppl.A3, The Chemistry of Double-Bonded Functional Groups, Ed. S. Patai, J. Wiley and Sons, 1997, 1113.
A. S. Morkovnik, L. N. Divaeva, and T. A. Kuz’menko, Izv. Akad. Nauk, Ser. Khim., 2006, 876 [Russ. Chem. Bull., Int. Ed., 2006, 55, 907]; (b) G. H. Hardtmann, G. Koletar, and O. R. Pfister, J. Med. Chem., 1975, 18, 447.
G. E. Hardtmann, US Pat. 4020062; http://v3.espacenet.com.
W. Forster and R. M. Laird, J. Chem. Soc., Perkin Trans. 2, 1982, 135.
A. Streitwieser, E. G. Jayasree, S. S.-H. Leung, and G. S.-C. Choy, J. Org. Chem., 2005, 70, 8486.
V. I. Minkin, A. D. Garnovskii, J. Elguero, A. R. Katritzky, and O. V. Denisko, Adv. Heterocycl. Chem., 2000, 76, 203.
F.-T. Hung, W.-P. Hu, T.-H. Li, C.-C. Cheng, and P.-T. Chou, J. Phys. Chem. A, 2003, 107, 3244.
D. Jacquemin, J. Preat, V. Wathelet, M. Fontaine, and E. A. Perpete, J. Am. Chem. Soc., 2006, 128, 2072.
J. K. Laerdahl and E. Uggerud, Int. J. Mass Spectrom., 2002, 214, 277.
A. Streitwieser, G. S.-C. Choy, and F. Abu-Hasanayn, J. Am. Chem. Soc., 1997, 119, 5013.
W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347.
K. K. Irikura, R. D. Johnson, III, and R. N. Kacker, J. Phys. Chem. A, 2005, 109, 8430.
T. Jen and B. Loev, US Pat. 3745216; http://v3.espacenet.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1150–1164, June, 2007.
Rights and permissions
About this article
Cite this article
Morkovnik, A.S., Divaeva, L.N. & Anisimova, V.A. Mechanism of the reaction of neutral and anionic N-nucleophiles with α-halocarbonyl compounds. Russ Chem Bull 56, 1194–1209 (2007). https://doi.org/10.1007/s11172-007-0182-1
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-007-0182-1