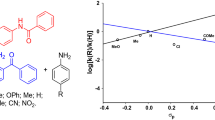
Abstract
Photoreduction of o-benzoquinones in the presence of para-substituted N,N-dimethyl-anilines under irradiation at λ ≥ 500 nm affords pyrocatechol monoethers of the 2-(amino-methoxy)phenol type. In the subsequent dark reaction, these monoethers undergo quantitative decomposition by a heterolytic mechanism to give the corresponding pyrocatechols and nitrogen-containing compounds. The rate of this decomposition decreases with decreasing size of the substituent at the position adjacent to the ether bond that is formed upon photoreduction. The redox characteristics of such pyrocatechol monoethers can serve as the criterion of their stability. A weakening of the electron-withdrawing properties of quinones and the electron-donating properties of amines leads to an increase in stability of their reaction products.
Similar content being viewed by others
References
A. Shonberg, Preparative organische Photochemie, Springer-Verlag, Berlin-Göttingen-Heidelberg, 1958.
S. Patai, The Chemistry of the Quinonoid Compounds, J. Wiley and Sons, London–New York–Sydney–Toronto, 1974, 616 p.
K. Maruyama and T. Otsuki, Bull. Chem. Soc. Jpn, 1971, 44, 2885.
K. Maruyama, T. Otsuki, H. Shindo, and T. Maruyama, Bull. Chem. Soc. Jpn, 1971, 44, 2000.
K. Maruyama, K. Ono, and T. Otsuki, Bull. Chem. Soc. Jpn, 1972, 45, 847.
A. I. Kryukov, V. P. Sherstyuk, and I. I. Dilung, Fotoperenos elektrona i ego prikladnye aspekty [Electron Phototransfer and Applied Aspects], Naukova dumka, Kiev, 1982, 239 pp. (in Russian).
S. Patai, The Chemistry of the Quinonoid Compounds, J. Wiley and Sons, Chichester-New York-Brisbane-Toronto-Singapore, 1988, 2, 878 p.
K. Maruyama, H. Shindo, and T. Maruyama, Bull. Chem. Soc. Jpn, 1971, 44, 585.
H. Shindo, K. Maruyama, T. Otsuki, and T. Maruyama, Bull. Chem. Soc. Jpn, 1971, 44, 2789.
K. Maruyama, T. Rwai, and I. Naruta, Bull. Chem. Soc. Jpn, 1978, 51, 2052.
M. Monroe and S. A. Weiner, J. Am. Chem. Soc., 1969, 91, 450.
E. Andrzejewska, L. Linden, and J. F. Rabek, Macromol. Chem. Phys., 1998, 199, 441.
G. A. Abakumov, S. A. Chesnokov, V. K. Cherkasov, and G. A. Razuvaev, Izv. Akad. Nauk SSSR, Ser. Khim., 1985, 773 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1985, 34, 700 (Engl. Transl.)].
S. A. Chesnokov, V. K. Cherkasov, G. A. Abakumov, Yu. A. Kurskii, M. P. Shurygina, O. N. Mamysheva, and A. S. Shavyrin, Izv. Akad. Nauk, Ser. Khim., 2003, 688 [Russ. Chem. Bull., Int. Ed., 2003, 52, 718].
C. A. Chesnokov, V. K. Cherkasov, Yu. V. Chechet, V. I. Nevodchikov, G. A. Abakumov, and O. N. Mamysheva, Izv. Akad. Nauk, Ser. Khim., 2000, 1515 [Russ. Chem. Bull., Int. Ed., 2000, 49, 1506].
Yu. V. Zefirov and P. M. Zorkii, Usp. Khim., 1995, 64, 446 [Russ. Chem. Rev., 1995, 64 (Engl. Transl.)].
K. Nakanishi, Infrared Absorption Spectroscopy, Holden-Day Inc., San Francisko and Nankodo Company Limited, Tokyo, 1962, p. 211.
G. M. Sheldrick, SHELXTL V. 6.12, Structure Determination Software Suite, Bruker AXS, Madison (Wisconsin, USA), 2000.
J. Gordon and R. A. Ford, The Chemist’s Companion, A Wiley Intercience Publication, J. Wiley and Sons, New York-London-Sydney-Toronto, 1972.
Weygand-Hilgetag, Organisch-chemische Experimentierkunst, Johann Ambrosios Barth, Leipzig, 1964, p. 464.
V. A. Garnov, V. I. Nevodchikov, G. A. Abakumov, L. G. Abakumova, Yu. A. Kurskii, and V. K. Cherkasov, Izv. Akad. Nauk SSSR, Ser. Khim., 1985, 2793 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1985, 34, 2589 (Engl. Transl.)]; (b) G. A. Abakumov, V. K. Cherkasov, L. G. Abakumova, and V. I. Nevodchikov, Izv. Akad. Nauk SSSR, Ser. Khim., 1990, 1098 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1990, 39, 984 (Engl. Transl.)]; (c) G. A. Abakumov, V. K. Cherkasov, L. G. Abakumova, V. I. Nevodchicov, N. O. Druzhkov, N. P. Makarenko, and Yu. A. Kursky, J. Organometal. Chem., 1995, 491, 127; (d) G. A. Abakumov, V. K. Cherkasov, L. G. Abakumova, N. O. Druzhkov, V. I. Nevodchikov, Yu. A. Kurskii, and N. P. Makarenko, Metalloorg. Khim., 1991, 4, 925 [Organomet. Chem. USSR, 1991, 4 (Engl. Transl.)]; (e) V. A. Garnov, V. I. Nevodchikov, L. G. Abakumova, G. A. Abakumov, and V. K. Cherkasov, Izv. Akad. Nauk SSSR, Ser. Khim., 1987, 1864 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1987, 36, 1728 (Engl. Transl.)].
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1528–1535, September, 2006.
Rights and permissions
About this article
Cite this article
Shurygina, M.P., Kurskii, Y.A., Chesnokov, S.A. et al. o-benzoquinone photoreduction products in the presence of N,N-dimethylanilines. Russ Chem Bull 55, 1585–1592 (2006). https://doi.org/10.1007/s11172-006-0458-x
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0458-x