Abstract
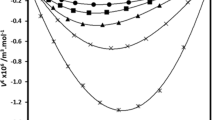
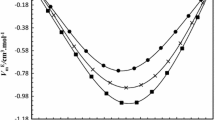
Densities of solutions of oleic, linoleic, and linolenic acids in n-hexane and n-heptane were measured using a vibrating-tube densimeter at 298.15 K in a concentration range of 0–0.012 molar fractions of solute. The measurement error does not exceed ±5·10−6 g cm−3. The limiting partial molar volumes of fatty acids of the studied series in n-hexane and n-heptane and the excess volume properties of binary mixtures were calculated. On going from oleic to linolenic acid, the number of double bonds (>C=C<) in a solute molecule increases, the hydrocarbon chain length in a solvent molecule decreases, and compactness of the structure packing of the resulting solution increases. This is caused, as a whole, by the enhancement of the n-alkane—acid intermolecular interaction.
Similar content being viewed by others
References
V. E. Vas’kovskii, Sorosovskii Obrazov. Zh. [Soros Educational J.], 1997, 3, 32 (in Russian).
D. G. Knorre and S. D. Myzina, Biologicheskaya khimiya [Biological Chemistry], Vysshaya Shkola, Moscow, 2000, p. 91 (in Russian).
N. M. Shilina and I. Ya. Kon’, Voprosy detskoi dietologii [Problems of Children’s Dietetics], 2004, 2, 25 (in Russian).
A. D. Buckland, C. H. Rochester, and S. A. Topham, J. Chem. Soc., Faraday Trans. 1, 1980, 76, 302.
O. M. Mikhailik, V. I. Povstugar, and S. S. Mikhailova, Zh. Prikl. Khim., 1992, 65, 1714 [Russ. J. Appl. Chem., 1992, 65 (Engl. Transl.)].
V. V. Korolev, A. G. Ramazanova, and A. V. Blinov, Izv. Akad. Nauk, Ser. Khim., 2002, 1888 [Russ. Chem. Bull., Int. Ed., 2002, 51, 2044].
V. V. Korolev, A. G. Ramazanova, V. I. Yashkova, O. V. Balmasova, and A. V. Blinov, Kolloid. Zh., 2004, 66, 779 [Colloid J., 2004, 66, 700 (Engl. Transl.)].
V. V. Korolev, A. V. Blinov, and A. G. Ramazanova, Kolloid. Zh., 2004, 66, 784 [Colloid J., 2004, 66, 705 (Engl. Transl.)].
A. F. Pshenichnikov and A. V. Lebedev, Kolloid. Zh., 2005, 67, 218 [Colloid J., 2005, 67 (Engl. Transl.)].
Yu. V. Karyakin and I. I. Angelov, Chistye khimicheskie veshchestva [Pure Chemical Substances], Khimiya, Moscow, 1974, 408 pp. (in Russian).
G. A. Krestov, V. N. Afanas’ev, and L. S. Efremova, Fizikokhimicheskie svoistva binarnykh rastvoritelei [Physicochemical Properties of Binary Solvents], Khimiya, Leningrad, 1986, 688 pp. (in Russian).
M. K. Kumaran, C. J Halpin, and G. C. Benson, J. Chem. Thermodyn., 1983, 15, 249.
A. Auceijo, M. C. Burguet, R. Muňoz, and J. L. Marques, J. Chem. Eng. Data, 1995, 40, 141.
M. K. Kumaran and G. C. Benson, J. Chem. Thermodyn., 1983, 15, 245.
V. K. Abrosimov and V. V. Korolev, in Eksperimental’nye metody khimii rastvorov: Densimetriya, viskozimetriya i drugie metody [Experimental Methods of Solution Chemistry: Densimetry, Viscosimetry, and Other Methods], Ed. A. M. Kutepov, Nauka, Moscow, 1997, p. 5 (in Russian).
E. V. Ivanov, E. Yu. Lebedeva, V. K. Abrosimov, and N. G. Ivanova, Izv. Akad. Nauk, Ser. Khim., 2004, 716 [Russ. Chem. Bull., Int. Ed., 2004, 53, 751].
V. V. Korolev, Zh. Fiz. Khim., 1989, 63, 1701 [Russ. J. Phys. Chem., 1989, 63 (Engl. Transl.)].
V. K. Abrosimov, Zh. Fiz. Khim., 1988, 62, 1913 [Russ. J. Phys. Chem., 1988, 62 (Engl. Transl.)].
V. S. Kuz’min and S. V. Katser, Izv. Akad. Nauk, Ser. Khim., 1992, 922 [Bull. Russ. Acad. Sci., Div. Chem. Sci., 1992, 41, 720 (Engl. Transl.)].
E. V. Ivanov, Zh. Fiz. Khim., 2004, 78, 1400 [Russ. J. Phys. Chem., 2004, 78, 1225 (Engl. Transl.)].
N. Brükl and J. I. Kim, Z. Phys. Chem. N. F., 1981, 126, 133.
V. A. Rabinovich and Z. Ya. Khavin, Kratkii khimicheskii spravochnik [Brief Chemical Handbook], Eds A. A. Potekhin and A. I. Efimov, Khimiya, Leningrad, 1991, 432 pp. (in Russian).
Y. Marcus, Ion Solvation, Wiley, Chichester, 1985, 306 pp.
R. Malhotra and L. A. Woolf, J. Chem. Thermodyn., 1991, 23, 49.
E. Wilhelm, R. Battino, and R. J. Wilcock, Chem. Rev., 1977, 77, 219.
V. F. Stolypin and A. I. Mishustin, Zh. Fiz. Khim., 1987, 61, 3226 [Russ. J. Phys. Chem., 1987, 61 (Engl. Transl.)].
M. Sakurai, Bull. Chem. Soc. Jpn, 1990, 63, 1695.
C. Klofutar and T. Nemec, J. Solut. Chem., 1996, 25, 1151.
C. L. De Ligny and H. G. Van der Veen, Chem. Eng. Sci., 1972, 27, 391.
Y. Maham, T. T. Teng, L. G. Hepler, and A. E. Mather, J. Solut. Chem., 1994, 23, 195.
B. Hawrylak, K. Gracie, and R. Palepu, J. Solut. Chem., 1998, 27, 17.
A. A. Tager and L. V. Adamova, Usp. Khim., 1980, 49, 618 [Russ. Chem. Rev., 1980, 49 (Engl. Transl.)].
V. P. Belousov and M. Yu. Panov, Termodinamika vodnykh rastvorov neelektrolitov [Thermodynamics of Aqueous Solutions of Nonelectrolytes], Khimiya, Leningrad, 1983, 265 pp. (in Russian).
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 643–647, April, 2006.
Rights and permissions
About this article
Cite this article
Ramazanova, A.G., Yashkova, V.I., Balmasova, O.V. et al. Volume properties of solutions of oleic, linoleic, and linolenic acids in n-hexane and n-heptane at 298.15 K. Russ Chem Bull 55, 666–671 (2006). https://doi.org/10.1007/s11172-006-0310-3
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0310-3