Abstract
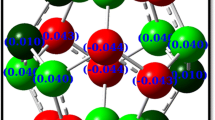
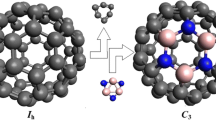
The geometric parameters and energy characteristics of small endofullerenes N@Cn (n = 20, 24, 30, 32, 40, 50) and N@C60 in the quartet ground state were calculated by the B3LYP/6-31G* method. The N atom is located at the center of the carbon cage in all molecules except N@C30, where it is bound to the cage wall. Encapsulation of nitrogen atom has little effect on the fullerene cage geometry for n = 40, 50, and 60. No significant charge transfer from the N endo-atom to the cage was revealed for all the N@Cn endofullerenes studied. The calculated spin density on the nitrogen endo-atom increases as the size (n) of the carbon cage increases. The relative stabilities of Cn fullerenes and corresponding endofullerenes N@Cn are discussed.
Similar content being viewed by others
References
Fullerenes: Chemistry, Physics and Technology, Eds K. M. Kadish and R. S. Ruoff, Wiley, New York, 2000.
A. Weidinger, M. Waiblinger, B. Pietzak, and T. A. Murphy, Appl. Phys. A, 1998, 66, 287.
C. Knapp, N. Weiden, H. Kass, K. P. Dinse, B. Pietzak, M. Waiblinger, and A. Weidinger, Mol. Phys., 1998, 95, 999.
A. Hirsch, in Top. Curr. Chem., Ed. A. Hirsch, Springer, Berlin—Heidelberg, 1999, 199, 1 (see also references cited therein).
J. C. Greer, Chem. Phys. Lett., 2000, 326, 567.
J. Lu, X. Zhang, and X. Zhao, Chem. Phys. Lett., 1999, 312, 85.
B. N. Plakhutin, N. N. Breslavskaya, E. V. Gorelik, and A. V. Arbuznikov, J. Mol. Struct. (THEOCHEM), 2005, 727, 149.
H. Prinzbach, A. Weiler, P. Landenberger, F. Wahl, J. Wörth, L. T. Scott, M. Gelmont, D. Olevano, and B. v. Issendorff, Nature, 2000, 407, 60.
C. Piskoti, J. Yarger, and A. Zettl, Nature, 1998, 393, 771.
M. Ata, H. Huang, and T. Akasaka, J. Phys. Chem. B, 2004, 108, 4640.
Z. Slanina and T. Chow, J. Nanosci. Nanotech., 2003, 3, 303.
W. Harneit, Phys. Rev. A, 2002, 65, 032322.
F. Elste and C. Timm, Phys. Rev. B Cond. Mat., 2005, 71, 155403.
N. N. Breslavskaya, A. A. Levin, and A. L. Buchachenko, Izv. Akad. Nauk, Ser. Khim., 2004, 19 [Russ. Chem. Bull., Int. Ed., 2004, 53 18].
A. L. Buchachenko, J. Phys. Chem. A, 2001, 105, 5839.
J. B. Foresman and E. Frish, Exploring Chemistry with Electronic Structure Methods, Gaussian Inc., Pittsburgh, 1996, 302 pp.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, GAUSSIAN-98, Revision A.9, Gaussian Inc., Pittsburgh (PA), 1998.
Z. Chen, H. Jiao, M. Bühl, A. Hirsch, and W. Thiel, Theor. Chem. Acc., 2001, 106, 352.
Z. Chen and W. Thiel, Chem. Phys. Lett., 2003, 367, 15.
S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 552.
P. W. Fowler and D. E. Manolopoulos, An Atlas of Fullerenes, Oxford Univ. Press, Oxford, 1995.
R. B. Darzynkiewicz and G. E. Scuseria, J. Phys. Chem. A, 1997, 101, 7141.
J. M. Hawkins, A. Meyer, T. A. Lewis, S. D. Loren, and F. J. Hollander, Science, 1991, 252, 312.
R. J. Cross, M. Saunders, and H. Prinzbach, Org. Lett., 1999, 1, 1479.
D. Moran, F. Stahl, E. D. Jemmis, H. F. Schlaefer, III, and P. v. R. Schleyer, J. Phys. Chem. A, 2002, 106, 5144.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 15–20, January, 2006.
Rights and permissions
About this article
Cite this article
Breslavskaya, N.N., Levin, A.A. & Buchachenko, A.L. Quantum chemical calculations of N@Cn endofullerenes (n ≤ 60). Russ Chem Bull 55, 16–21 (2006). https://doi.org/10.1007/s11172-006-0209-z
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0209-z