Abstract
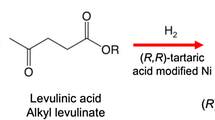
The rate of hydrogenation of γ-ketoesters MeCOCH2CH2COOR (R = Et, Pri, But) in the presence of the chiral RuII—BINAP catalyst (BINAP is 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) greatly increases upon the addition of 5–10 equivalents of HCl with respect to ruthenium. In the hydrogenation of ethyl levulinate, the optically active γ-hydroxy ester initially formed would cyclize by ∼95% to give γ-valerolactone with optical purity of 98–99% ee. When the Ru(COD)(MA)2—BINAP—HCl catalytic system is used (COD is 1,5-cyclooctadiene, MA is 2-methylallyl), complete conversion of the ketoester (R = Et) in EtOH is attained in 5 h at 60 °C under an H2 pressure of 60–70 atm.
Similar content being viewed by others
References
T. Naota, H. Takaya, and S.-I. Murahashi, Chem. Rev., 1998, 98, 2599.
D. J. Ager and S. A. Laneman, Tetrahedron Asymmetry, 1997, 8, 3327.
R. Noyori, Acta Chem. Scand., 1996, 50, 380.
K. Mashima, K. Kusano, N. Sato, Y. Matsumura, K. Nozaki, H. Kumobayashi, N. Sayo, Y. Hori, T. Ishizaki, S. Akutagawa, and H. Takaya, J. Org. Chem., 1994, 59, 3064.
W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3045.
T. Ohkuma, M. Kitamura, and R. Noyori, Tetrahedron Lett., 1990, 31, 5509.
K. Tohdo, Y. Hamada, and T. Shioiri, Synlett, 1994, 105.
S. A. King, J. Org. Chem., 1994, 59, 2253.
E. L. Stangeland and T. Sammakia, J. Org. Chem., 2004, 69, 2381.
G. Juszkiewicz, M. Asztemborska, and J. Jurczak, Pol. J. Chem., 2002, 76, 1707.
T. Saito, T. Yokozawa, T. Ishizakis, T. Moroi, N. Sayo, T. Miura, and H. Kumobayashi, Adv. Synth. Catal., 2001, 343, 264.
J. P. Genet, C. Pinel, V. Ratovelomanana-Vidal, S. Mallart, X. Pfister, L. Bischoff, M. C. Cano De Andrade, S. Darses, C. Galopin, and J. A. Laffitte, Tetrahedron Asymmetry, 1994, 5, 675.
S. A. King, A. S. Thompson, A. O. King, and T. R. Verhoeven, J. Org. Chem., 1992, 57, 6689.
M. Kitamura, M. Yoshimura, N. Kanda, and R. Noyori, Tetrahedron, 1999, 55, 8769.
V. A. Pavlov, E. V. Starodubtseva, M. G. Vinogradov, V. A. Ferapontov, O. R. Malyshev, and G. L. Heise, Izv. Akad. Nauk. Ser. Khim., 2000, 725 [Russ. Chem. Bull., 2000, 49, 728 (Engl. Transl.)].
R. Noyori and T. Ohkuma, Andgew. Chem., Int. Ed. Engl., 2001, 40, 41.
A. Mezzetti, A. Tschumper, and G. Consiglio, J. Chem. Soc., Dalton Trans., 1995, 49.
F. Maienza, F. Santoro, F. Spindler, C. Malan, and A. Mezzetti, Tetrahedron Asymmetry, 2002, 13, 1817.
K. Mashima, T. Hino, and H. Takaya, Tetrahedron Lett., 1991, 32, 3101.
M. Kinoshita and S. Umezawa, Bull. Chem. Soc. Jpn, 1970, 43, 897.
R. A. Zelonka and M. C. Baird, Can. J. Chem., 1972, 50, 3063.
H. Ikeda, E. Sato, T. Sugai, and H. Ohta, Tetrahedron, 1996, 52, 8113.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 2301–2304, October, 2005.
Rights and permissions
About this article
Cite this article
Starodubtseva, E.V., Turova, O.V., Vinogradov, M.G. et al. Enantioselective hydrogenation of levulinic acid esters in the presence of the RuII-BINAP-HCl catalytic system. Russ Chem Bull 54, 2374–2378 (2005). https://doi.org/10.1007/s11172-006-0125-2
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0125-2