Abstract
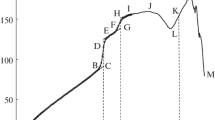
The temperature dependence of the heat capacity of an alternating copolymer of bicyclo[2.2.1]hepta-2,5-diene and carbon monoxide in the temperature range 6–550 K (with an error of 0.2–0.5% at 6–350 K and 0.5–1.5% at 330–550 K) was studied by the adiabatic vacuum and dynamic calorimetry. Physical transformations of the copolymer in the studied temperature region were identified, and their thermodynamic characteristics were determined. The combustion energy of the copolymer at 298.15 K was measured in a calorimeter with a static bomb and isothermal jacket. The thermodynamic functions for a region of 0–550 K, enthalpy of combustion, and thermodynamic parameters of copolymer formation from simple substances at T = 298.15 K and p = 101.325 kPa were calculated from the obtained experimental data. The new results and earlier published data were used for the calculation of the thermodynamic characteristics of the alternate copolymerization of bicyclo[2.2.1]hepta-2,5-diene and CO under standard pressure for a region of 0–350 K for the bulk reaction.
Similar content being viewed by others
References
B. V. Lebedev, K. B. Zhogova, Ya. V. Denisova, G. P. Belov, and O. N. Golodkov, Izv. Akad. Nauk, Ser. Khim., 1998, 284 [Russ. Chem. Bull., 1998, 47, 277 (Engl. Transl.)].
B. V. Lebedev, A. V. Tsvetkova, N. N. Smirnova, G. P. Belov, O. N. Golodkov, and Yu. A. Kurskii, Izv. Akad. Nauk, Ser. Khim., 1999, 1527 [Russ. Chem. Bull., 1999, 48, 1507 (Engl. Transl.)].
A. V. Arapova, B. V. Lebedev, N. N. Smirnova, T. G. Kulagina, G. P. Belov, and O. N. Golodkov, Izv. Akad. Nauk, Ser. Khim., 2001, 2264 [Russ. Chem. Bull., Int. Ed., 2001, 50, 2372].
T. A. Bykova, N. N. Smirnova, G. P. Belov, and E. V. Novikova, Vysokomol. Soedin., Ser. B, 2004, 46, 374 [Polym. Sci., Ser. B, 2004, 46 (Engl. Transl.)].
A. Sen, Chemtech., 1986, 48.
G. P. Belov, Vysokomol. Soedin., Ser. B, 1998, 40, 508 [Polym. Sci., Ser. B, 1998, 40 (Engl. Transl.)].
N. S. Enikolopyan and P. E. Matkovskii, Neorganicheskie gazoobraznye okisly kak somonomery [Inorganic Gaseous Oxides as Comonomers], Nauka, Moscow, 1985, 85 pp. (in Russian).
G. P. Belov, Vysokomol. Soedin., Ser. B, 2001, 43, 1651 [Polym. Sci., Ser. B, 2001, 43 (Engl. Transl.)].
G. P. Belov, Izv. Akad. Nauk, Ser. Khim., 2002, 1475 [Russ. Chem. Bull., Int. Ed., 2002, 51, 1605].
E. V. Novikova, G. P. Belov, W. Klaui, and A. A. Solotnov, Vysokomol. Soedin., Ser. A, 2003, 45, 1605 [Polym. Sci., Ser. A, 2003, 45 (Engl. Transl.)].
R. M. Varushchenko, A. I. Druzhinina, and E. L. Sorkin, J. Chem. Thermodyn., 1997, 29, 623.
V. M. Malyshev, G. A. Mil'ner, E. L. Sorkin, and V. F. Shibakin, Pribory i tekhnika eksperimenta, 1985, No. 6, 195 [Instr. Exp. Techn., 1985, No. 6 (Engl. Transl.)].
B. V. Lebedev and E. G. Kiparisova, Zh. Fiz. Khim., 1996, 70, 1351 [Russ. J. Phys. Chem., 1996, 70 (Engl. Transl.)].
A. G. Kabo and V. V. Diky, Thermochim. Acta, 2000, 347, 79.
S. Alford and M. Dole, J. Chem. Soc., 1955, 77, 4774.
G. Adam and J. H. Gibbs, J. Chem. Phys., 1965, 43, 139.
T. S. Yakubov, Dokl. Akad. Nauk SSSR, 1990, 310, 145 [Dokl. Chem., 1990 (Engl. Transl.)].
A. D. Izotov, O. V. Shebershneva, and K. S. Gavrichev, Trudy Vsesoyuzn. konf. po termicheskomu analizu i kalorimetrii [Proceedings of the All-Russian Conf. on Thermal Analysis and Calorimetry], Kazan, 1996, 200 (in Russian).
V. V. Tarasov, Zh. Fiz. Khim., 1952, 26, 1374 [Russ. J. Phys. Chem., 1952, 26 (Engl. Transl.)].
A. Bestul and S. S. Chang, J. Chem. Phys., 1964, 40, 3731.
B. V. Lebedev, Thermochim. Acta, 1997, 297, 143.
Termicheskie konstanty veshchestv [Thermal Constants of Substances], Ed. V. P. Glushko, Izd-vo VINITI, Moscow, 1965–1982, Issues 1–6 (in Russian).
Termodinamicheskie svoistva individual'nykh veshchestv [Thermodynamic Properties of Individual Substances], Ed. V. P. Glushko, Nauka, Moscow, 1979, Vol. 2, book 2 (in Russian).
W. T. Steel, J. Chem. Thermodyn., 1978, 10, 919.
T. A. Bykova, N. N. Smirnova, L. V. Nikishchenkova, G. P. Belov, and E. V. Novikova, Zh. Fiz. Khim., 2004, 78, 1944 [Russ. J. Phys. Chem., 2004, 78 (Engl. Transl.)].
F. S. Dianton and K. J. Ivin, Quart. Revs. London Chem. Soc., 1958, 12, 61.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1483–1487, June, 2005.
Rights and permissions
About this article
Cite this article
Bykova, T.A., Smirnova, N.N., Kulagina, T.G. et al. Thermodynamic properties of an alternating copolymer of bicyclo[2.2.1]hepta-2,5-diene and carbon monoxide in a region from T → 0 to 550 K. Russ Chem Bull 54, 1527–1531 (2005). https://doi.org/10.1007/s11172-005-0441-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0441-y