Abstract
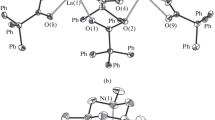
The reactions of dipotassium and disodium salts of the tetraphenylethylene dianion with LuCl3(THF)3 or CpLuCl2(THF)3 yielded the homoleptic ate-complexes [Na(THF)5][Lu(Ph2CCPh2)2] (1) and [K(THF)5][Lu(Ph2CCPh2)2] (2) or the heteroleptic complex CpLu(Ph2CCPh2)(THF)2 (4), respectively. Recrystallization of complex 1 from a diglyme—THF mixture afforded [Na(diglyme)2][Lu(Ph2CCPh2)2](THF)0,5 (3). Recrystallization of complex 4 from 1,2-dimethoxyethane gave [CpLu(Ph2CCPh2)(DME)](DME) (5). The structures of complexes 3 and 5 were established by X-ray diffraction analysis. In both complexes, the unusual η6-coordination of the (Ph2CCPh2)2− dianion to lutetium is observed. The Lu-C distances vary from 2.441(2) to 2.643(2) Å (3) and from 2.470(3) to 2.763(3) Å (5). In complexes 3 and 5, a redistribution of the C-C bond lengths was observed in the Ph groups coordinated to lutetium. Studies by 1H, 13C, and 2D NMR spectroscopy demonstrated that the η6-coordination of the tetraphenylethylene dianion in homoleptic ate-complexes 1 and 2 is retained in a THF solution, whereas the coordination of this dianion in heteroleptic complex 4 changes from η6 to η4.
Similar content being viewed by others
References
F. T. Edelman, in Comprehensive Organometallic Chemistry II; Vol. 4, Eds E. W. Abel, F. G. A. Stone, and G. Wilkinson, Pergamon, Oxford, 1995, Ch. 2; R. Anwander and W. A. Herrmann, Top. Curr. Chem., 1996, 179, 1; W. J. Evans, Coord. Chem. Rev., 2000, 206–207, 263; M. F. Lappert, J. Organomet. Chem., 2000, 600, 144; (b) F. T. Edelmann, D. M. M. Freckmann, and H. Schumann, Chem. Rev., 2002, 102, 1851; M. N. Bochkarev, Chem. Rev., 2002, 102, 2089.
W. J. Evans, New J. Chem., 1995, 19, 525; F. Nief, Eur. J. Inorg. Chem., 2001, 891; S. Arndt and J. Okuda, Chem. Rev., 2002, 102, 1953; W. J. Evans an B. L. Davis, Chem. Rev., 2002, 102, 2119.
W. J. Evans, Polyhedron, 1987, 6, 803.
R. G. Hayes and J. L. Thomas, J. Am. Chem. Soc., 1969, 91, 6876; F. Mares, K. Hodgson, and A. Streitwieser, Jr., J. Organomet. Chem., 1970, 24, C68; U. Kilimann, R. Herbst-Irner, D. Sralke, and F. T. Edelmann, Angew. Chem., Int. Ed. Engl., 1994, 33, 1618.
M. J. Manning, C. B. Knobler, and M. F. Hawthorne, J. Am. Chem. Soc., 1988, 110, 4458; R. Khattar, C. B. Knobler, S. E. Johnson, and M. F. Hawthorne, Inorg. Chem., 1991, 30, 1970; M. J. Manning, C. B. Knobler, R. Khattar, and M. F. Hawthorne, Inorg. Chem., 1991, 30, 2009; N. S. Hosmane, D. Zhu, H. Zhang, A. R. Oki, and J. A. Maguire, Organomet., 1998, 17, 3196; Z. Xie, S. Wang, Z.-Y. Zhou, F. Xue, and T. C. W. Mak, Organomet., 1998, 17, 489; K. Chiu, Z. Zhang, T. C. W. Mak, and Z. Xie, J. Organomet. Chem., 2000, 614, 107; Z. Xie, K. Chui, Q. Yang, and T. C. W. Mak, Organomet., 1999, 18, 3947; S. Wang, H.-W. Li, and Z. Xie, Organomet., 2001, 20, 3842.
A. V. Protchenko, L. N. Zakharov, M. N. Bochkarev, and Yu. T. Struchkov, J. Organomet. Chem., 1993, 447, 209; A. V. Protchenko, O. G. Almazova, L. N. Zakharov, G. K. Fukin, Yu. T. Struchkov, and M. N. Bochkarev, J. Organomet. Chem., 1997, 536/537, 457; M. N. Bochkarev, I. L. Fedushkin, A. A. Fagin, H. Schumann, and J. Demtschuk, Chem. Commun., 1997, 1783; I. L. Fedushkin, M. N. Bochkarev; H. Schumann, L. Esser, and G. Kociok-Kohn, J. Organomet. Chem., 1995, 489, 145; W. J. Evans, N. T. Allen, and J. W. Ziller, J. Am. Chem. Soc., 2000, 122, 11749.
D. M. Roitershtein, A. M. Ellern, M. Yu. Antipin, L. F. Rybakova, Yu. T. Struchkov, and E. S. Petrov, Mendeleev Commun., 1992, 118; (b) D. M. Roitershtein, L. F. Rybakova, E. S. Petrov, A. M. Ellern, M. Yu. Antipin, and Yu. T. Struchkov, J. Organomet. Chem., 1993, 460, 39; (c) W. J. Evans, S. L. Gonzales, and W. J. Ziller, J. Am. Chem. Soc., 1994, 116, 2600; (d) K.-H. Thiele, S. Bambirra, H. Schumann, and H. Hemling, J. Organomet. Chem., 1996, 517, 161.
J. Scholz, A. Scholz, R. Weimann, C. Janiak, and H. Schumann, Angew. Chem., Int. Ed. Engl., 1994, 33, 1171.
K. H. Thiele, S. Bambirra, J. Sieler, and S. Yelonek, Angew. Chem., Int. Ed. Engl., 1998, 37, 2886; M. C. Cassani, Yu. K. Gun’ko, P. B. Hitchcock, M. F. Lappert, and F. Laschi, Organomet., 1999, 18, 5539.
D. M. Roitershtein, J. W. Ziller, and W. J. Evans, J. Am. Chem. Soc., 1998, 120, 11342.
M. Szwarc, Carbanions. Living Polymer and Electron Transfer Processes, Interscience, New York, 1968.
M. Szwarc, Ions and Ion Pairs in Organic Reactions, Interscience, New York, 1972.
A. Hoekstra and A. Vos, Acta Crystallogr., Sect. B, 1975, 31, 1716.
H. Bock, T. Hauck, and C. Nather, Organomet., 1996, 15, 1527.
H. Schumann and A. Dietrich, J. Organomet. Chem., 1991, 401, C33.
M. B. Zielinski, D. K. Drummond, P. S. Iyer, J. T. Leman, and W. J. Evans, Organomet., 1995, 14, 3724.
S. L. Latesky, A. K. McMullen, G. P. Niccolai, I. P. Rothwell, and J. C. Huffman, Organomet., 1985, 4, 902.
F. A. Cotton and M. D. La Prade, J. Am. Chem. Soc., 1968, 90, 5418.
U. Behrens and E. Weiss, J. Organomet. Chem., 1975, 96, 399.
U. Behrens and E. Weiss, J. Organomet. Chem., 1975, 96, 435.
J. R. Bleeke, R. R. Burch, C. L. Coulman, and B. C. Schardt, Inorg. Chem., 1981, 20, 1316.
R. R. Burch, E. L. Muetterties, and V. W. Day, Organomet., 1982, 1, 188.
A. Sonoda, P. M. Bailey, and P. M. Maitlis, J. Chem. Soc., Dalton Trans., 1979, 346.
E. A. Mintz, K. G. Moloy, T. J. Marks, and V. W. Day, J. Am. Chem. Soc., 1982, 104, 4692.
P. G. Edwards, R. A. Andersen, and A. Zalkin, Organomet., 1984, 3, 293.
M. Boij, A. Meetsma, and J. H. Teuben, Organomet., 1991, 10, 3246.
W. J. Evans, T. A. Ulibarri, and J. W. Ziller, J. Am. Chem. Soc., 1990, 112, 219.
W. J. Evans, D. K. Drummond, L. R. Chamberlain, R. J. Doedens, S. G. Bott, H. Zhang, and J. L. Atwood, J. Am. Chem. Soc., 1988, 110, 4983.
S. Frosen and R. A. Hoffman, Acta Chem. Scand., 1963, 1787.
J. Y. Noggle and R. E. Shirmer, The Nuclear Overhauser Effect Chemical Applications, New York, Academic, New York, 1971.
B. Gabor, K. Jonas, and K. Mynott, Inorg. Chim. Acta, 1998, 270, 555; H. Windisch, J. Scholz, R. Taube, and B. Wrackmeyer, J. Organomet. Chem., 1996, 520, 23.
A. Domenciano, P. Murray-Rust, and A. Vaciago, Acta. Cryst. Sec. B, 1983, B39, 457; D. Hoffmann, W. Bauer, P. R. Schleyer, U. Pieper, and D. Stalke, Organomet., 1993, 12, 1193; D. Hoffmann, W. Bauer, F. Hampel, N. J. R. E. Hommes, P. R. Schleyer, P. Otto, U. Pieper, D. Stalke, D. S. Wright, and R. Snaith, J. Am. Chem. Soc., 1994, 116, 528.
W. A. Herrmann, in Synthetic Methods of Organometallic and Inorganic Chemistry, Vol. 6 Lanthanides and Actinides; Ed. F. T. Edelmann, Verlag, Stuttgart, 1997, 34.
S. Manastyrskyj, R. E. Maginn, and M. Dubeck, Inorg. Chem., 1963, 2, 904.
Author information
Authors and Affiliations
Additional information
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 2060–2068, October, 2004.
Rights and permissions
About this article
Cite this article
Roitershtein, D.M., Minyaev, M.E., Lyssenko, K.A. et al. Lutetium complexes with tetraphenylethylene dianion. Synthesis and structure. Russ Chem Bull 53, 2152–2161 (2004). https://doi.org/10.1007/s11172-005-0089-7
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0089-7