Abstract
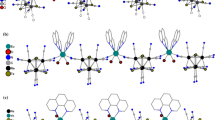
The reactions of anionic molybdenum and tungsten cyanide cuboidal clusters with CuII and MnII salts afforded two new cyanide-bridged heterometallic coordination polymers with the composition [{Cu2(dien)2(CN)}2{Mo4Te4(CN)12}]⋅14.5H2O (1) and (H3O)3K[{Mn(H2O)2}2{Mn(H2O)2(NO3)}4{W4Te4(CN)12}2]·8H2O (2). The structures of these compounds were established by X-ray diffraction analysis. Compound 1 has a layered structure, in which the cuboidal cluster fragments {Mo4Te4(CN)12}6− are linked to the copper atoms of the dinuclear fragments {(H2O)(dien)Cu(μ-CN)Cu(dien)(H2O)} through the bridging CN groups. Coordination polymer 2 has a framework structure, in which the cluster fragments {W4Te4(CN)12}6− are linked to the manganese(II) aqua complexes of two types, viz., the dinuclear fragment {Mn(μ2-H2O)2Mn} and the tetranuclear cyclic fragment {(H2O)2Mn(μ2-NO3)}4, through the bridging CN groups.
Similar content being viewed by others
References
K. R. Dunbar and R. A. Heintz, Prog. Inorg. Chem., 1997, 45, 283.
T. Iwamoto, in Comprehensive Supramolecular Chemistry, Eds J. L. Atwood, J. E. D. Davies, D. D. Macnicol, and F. Vogtle, Pergamon, 1996, 6, 643.
B. H. Chadwick and A. G. Sharpe, Adv. Inorg. Chem. Radiochem., 1966, 8, 83.
I. V. Tananaev, G. B. Seifer, Yu. Ya. Kharitonov, V. G. Kuznetsov, and A. P. Korol’kov, Khimiya ferrotsianidov [Chemistry of Ferrocyanides], Nauka, Moscow, 1971, 320 pp. (in Russian).
N. G. Naumov, A. V. Virovets, and V. E. Fedorov, Zh. Strukt. Khim., 2000, 41, 609 [Russ. J. Struct. Chem., 2000, 41 (Engl. Transl.)].
N. G. Naumov, A. V. Virovets, M. N. Sokolov, S. B. Artemkina, and V. E. Fedorov, Angew. Chem., Int. Ed., 1998, 37, 1943.
N. G. Naumov, S. B. Artemkina, A. V. Virovets, and V. E. Fedorov, J. Solid State Chem., 2000, 153, 195.
N. G. Naumov, A. V. Virovets, and V. E. Fedorov, Inorg. Chem. Commun., 2000, 3, 71.
M. P. Shores, L. G. Beauvais, and J. R. Long, J. Am. Chem. Soc., 1999, 121, 775.
Yu. V. Mironov, A. V. Virovets, S. B. Artemkina, and V. E. Fedorov, Angew. Chem., Int. Ed., 1998, 37, 2507.
Yu. V. Mironov, A. V. Virovets, W. S. Sheldrick, and V. E. Fedorov, Polyhedron, 2000, 20, 969.
Yu. V. Mironov, V. E. Fedorov, I. Ijjaali, and J. A. Ibers, Inorg. Chem., 2001, 40, 6320.
Yu. V. Mironov, O. A. Efremova, D. Yu. Naumov, W. S. Sheldrick, and V. E. Fedorov, Eur. J. Inorg. Chem., 2003, 2591.
V. P. Fedin, I. V. Kalinina, A. V. Virovets, N. V. Podberezskaya, and A. G. Sykes, Chem. Commun., 1998, 233.
V. P. Fedin, I. V. Kalinina, D. G. Samsonenko, Yu. V. Mironov, M. N. Sokolov, S. V. Tkachev, A. V. Virovets, N. V. Podberezskaya, and A. G. Sykes, Inorg. Chem., 1999, 38, 1956.
V. P. Fedin, D. G. Samsonenko, A. V. Virovets, I. V. Kalinina, and D. Yu. Naumov, Izv. Akad. Nauk, Ser. Khim., 2000, 18 [Russ. Chem. Bull., Int. Ed., 2000, 49, 19].
M. R. J. Elsegood, A. V. Virovets, D. G. Samsonenko, I. V. Kalinina, and V. P. Fedin, Zh. Strukt. Khim., 2000, 41, 1290 [Russ. J. Struct. Chem., 2000, 41 (Engl. Transl.)].
V. P. Fedin, I. V. Kalinina, A. V. Gerasimenko, and A. V. Virovets, Inorg. Chim. Acta, 2002, 331, 48.
V. P. Fedin, I. V. Kalinina, A. V. Virovets, and D. Fenske, Izv. Akad. Nauk, Ser. Khim., 2003, 119 [Russ. Chem. Bull., Int. Ed., 2003, 52, 126].
V. P. Fedin, A. V. Virovets, I. V. Kalinina, V. N. Ikorskii, M. R. J. Elsegood, and W. Clegg, Eur. J. Inorg. Chem., 2000, 2341.
V. P. Fedin, I. V. Kalinina, A. V. Virovets, and D. Fenske, Izv. Akad. Nauk, Ser. Khim., 2001, 1451 [Russ. Chem. Bull., Int. Ed., 2001, 50, 1525].
I. V. Kalinina, D. G. Samsonenko, V. A. Nadolinnyi, J. Lipkowski, and V. P. Fedin, Izv. Akad. Nauk, Ser. Khim., 2004, 86 [Russ. Chem. Bull., Int. Ed., 2004, 53, 86].
G. M. Sheldrick, SHELXTL v. 5.1 Software Reference Manual, Bruker AXS Inc., Madison, Wisconsin (USA), 1997.
G. M. Sheldrick, SHELX 97. Release 97-2, Gottingen University, Gottingen (Germany), 1998.
Author information
Authors and Affiliations
Additional information
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 2045–2050, October, 2004.
Rights and permissions
About this article
Cite this article
Kalinina, I.V., Samsonenko, D.G., Gerasimenko, A.V. et al. Cluster cyanide-bridged heterometallic coordination polymers: synthesis and crystal structures of compounds [{Cu2(dien)2(CN)}2{Mo4Te4(CN)12}]·14.5H2O and (H3O)3K[{Mn(H2O)2}2{Mn(H2O)2(NO3)}4{W4Te4(CN)12}2]·8H2O. Russ Chem Bull 53, 2135–2141 (2004). https://doi.org/10.1007/s11172-005-0086-x
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0086-x