Abstract
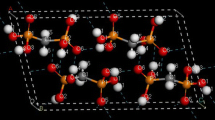
The equilibrium geometries, vibrational frequencies, and IR band intensities were calculated for the monomer and hydrogen-bonded cyclic dimer of ε-caprolactam (1) by the density functional B3LYP/6-311++G(d,p) method and compared with the experimental data. The gas phase IR spectrum of monomer of 1 was first measured. The calculated hydrogen bonding enthalpy ΔH/2 in the hydrogen-bonded dimer in the gas phase (–5.93 kcal/mol) is consistent with the published data. The computed scaled (scaling factor 0.97) vibrational frequencies of the monomer and dimer are in good agreement with the experimental data. The geometry of the ε-caprolactam monomer remains nearly unchanged in its dimer except for the N-H, C-O, and C-N bond lengths that respectively change from 1.012, 1.230, and 1.369 Å in the former to 1.029, 1.246, and 1.350 Å in the latter. The frequencies, eigenvectors, and IR intensities of the amide modes of the monomer and dimer differ dramatically. The calculated νNH and νCO frequency shifts due to hydrogen bonding are in good agreement with the experimental data, but theoretical intensification of the νNH IR band is much greater than that observed experimentally (by nearly 69 times vs. 11 times, respectively). The calculated N...O intermolecular distance in the structure of ε-caprolactam dimer equals the experimental value (2.89 Å). The influence of the basis set employed on the results of calculations is discussed.
Similar content being viewed by others
References
Yu. N. Panchenko, Izv. Akad. Nauk, Ser. Khim., 1996, 800 [Russ. Chem. Bull., 1996, 45, 753 (Engl. Transl.)].
J. B. Foresman and A. E. Frisch, Exploring Chemistry with Electronic Structure Methods, Pittsburg (PA), 1996, Ch. 4, p. 64.
J. Palomar, J. L. G. De Paz, and J. Catalan, Chem. Phys., 1999, 246, 167
S. Scheiner, Hydrogen bonding. A Theoretical perspective, Oxford University Press, New York—Oxford, 1997 (a) Ch. 1.3.2; (b) Chs. 1.5 and 1.6.
S. K. Madan and J. A. Sturr, Inorg. Chem., 1967, 6, 421.
Kh. Kh. Khakimov, in Reaktsionnaya sposobnost’ koordinatsionnykh soedinenii [Reactivity of Coordination Compounds], Nauka, Moscow, 1976, p. 156 (in Russian).
A. Aarna, K. Kijsler, and P. Krist’yanson, in Tr. I Vses. Konf. po Kleyam i Tekhnologii Skleivaniya [Proc. Ist All-Union Conf. on Glues and Glueing Technology], Izd. Tallin Polytechnic Institute, Tallin, 1966, p. 43 (in Russian).
A. Aarna, P. Krist’yanson, and Kh. Oya, Izv. Akad. Nauk Est. SSR, Khim. Geol. [Bull. Acad. Sci. Est. SSR. Chem. and Geol. Series], 1970, 19, 121 (in Russian).
F. K. Winkler and J. D. Dunitz, Acta Crystallogr., 1973, B29, 154.
Kh. P. Oya and R. M. Myasnikova, Zh. Strukt. Khim., 1974, 15, 679 [J. Struct. Chem. USSR, 1974, 15 (Engl. Transl.)].
C. Y. S. Chen and C. A. Swenson, J. Phys. Chem., 1989, 73, 2999.
A. V. Iogansen, G. A. Kurchi, and B. V. Rassadin, Zh. Prikl. Spektroscopii., 1969, 11, 1055 [J. Appl. Spectr. USSR, 1969, 11 (Engl. Transl.)].
A. V. Iogansen, Spectrochimica Acta, Part A, 1999, 55, 1585.
L. A. Dement’ev, A. V. Iogansen, and G. A. Kurchi, Opt. i Spektrosk [Optics and Spectroscopy], 1970, 29, 868 (in Russian).
M. Laznewski and J. Mokrzan, Z. Phys. Chem., 1967, 235.
R. C. Lord and T. J. Porro, Z. Elektrochem., 1960, 64, 672.
A. Aihara, Bull. Chem. Soc. Jpn, 1960, 33, 1188.
B. Schrader, Raman/Infrared Atlas of Organic Compounds, 2nd ed., VCH, 1989, I16–02.
T. I. Novikova, L. G. Kovalenko, N. V. Labinskaya, and Yu. N. Nizel’skii, Ukr. Khim. Zh. [Ukr. Chem. J.], 1982, Issue 10, 48 (in Russian).
A. Warshel, M. Levitt, and S. Lifson, J. Mol. Spectrosc., 1970, 33, 84.
L. J. Bellamy, The Infra-Red Spectra of Complex Molecules, Wiley, New York; Methuen and Co., London, 1957.
N. E. Triggs and J. J. Valentini, J. Phys. Chem., 1992, 96, 6922.
N. E. Triggs, R. T. Bonn, and J. J. Valentini, J. Phys. Chem., 1993, 97, 5535.
Kh. P. Oya and R. M. Myasnikova, Zh. Strukt. Khim., 1973, 14, 1094 [J. Struct. Chem. USSR, 1973, 14 (Engl. Transl.)].
G. Pimentel and A. L. McClellan, The Hydrogen Bond, Ed. L. Pauling, San Francisco—London, 1960.
A. Winkler and P. Hess, J. Am. Chem. Soc., 1994, 116, 9233.
V. A. Sipachev, J. Mol. Struct. (Theochem.), 1985, 121, 143.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1818–1825, September, 2004.
Rights and permissions
About this article
Cite this article
Garbuzova, I.A., Lokshin, B.V. Hydrogen bonding in ε-caprolactam dimer: a quantum-chemical study. Russ Chem Bull 53, 1894–1902 (2004). https://doi.org/10.1007/s11172-005-0047-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0047-4