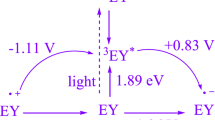
Abstract
A green radical synthetic approach for the production of tetrahydrobenzo[b]pyran scaffolds, which utilizes a Knoevenagel–Michael cyclocondensation reaction of aldehydes, malononitrile, and dimedone, has been devised. This innovative technique has been designed to achieve environmental sustainability. A novel single-electron transfer photocatalyst was employed for the synthesis in an aqueous ethanol solution under an air atmosphere at room temperature and stimulated with blue LED illumination serving as a renewable energy source. The objective of this undertaking is to cultivate a metal-free donor–acceptor (D–A) photocatalyst that is highly affordable and universally accessible. 9-Mesityl-10-methylacridinium perchlorate (Mes-Acr-Me+ClO4−) is recognized for its expeditious and effortless applicability, high efficiency in yielding products, low energy consumption, and commendable eco-friendliness. This capability facilitates the investigation into the temporal alterations of environmental and chemical constituents. A research inquiry was conducted with the primary objective of determining the turnover number and turnover frequency associated with tetrahydrobenzo[b]pyran scaffolds. Furthermore, the attainment of cyclization at a gram-scale level offers substantiation for its feasibility as a viable solution for industrial implementation.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and Supporting Information file.
References
F. Mohamadpour, Catal. Surv. Asia 27, 306 (2023)
F. Mohamadpour, Sci. Rep. 13, 13142 (2023)
F. Mohamadpour, J. Chem. Sci. 135, 74 (2023)
F. Mohamadpour, RSC Adv. 13, 2514 (2023)
R. Bhanja, S.K. Bera, P. Mal, Chem. Comm. 59, 4455 (2023)
S. Fukuzumi, H. Kotani, K. Ohkubo, S. Ogo, N.V. Tkachenko, H. Lemmetyinen, J. Am. Chem. Soc. 126, 1600 (2004)
M.M. Brasholz, Acridinium dyes and quinones in photocatalysis in science of synthesis photocatalysis in organic synthesis, ed. by B. Kçnig (Georg Thieme Verlag, Stuttgart, 2019)
A. Joshi-Pangu, F. Lévesque, H.G. Roth, S.F. Oliver, L.C. Campeau, D. Nicewicz, D.A. DiRocco, J. Org. Chem. 81, 7244 (2016)
A. Tlili, S. Lakhdar, Angew. Chem. 133, 19678 (2021)
F. Mohamadpour, J. Saudi Chem. Soc. 24, 636 (2020)
F. Mohamadpour, J. Photochem. Photobiol. A Chem. 407, 113041 (2021)
F. Mohamadpour, Monatsh. Chem. 152, 507 (2021)
N. Foloppe, L.M. Fisher, R. Howes, A. Potter, A.G.S. Robertson, A.E. Surgenor, Bioorg. Med. Chem. 14, 4792 (2006)
S.C. Kuo, L.J. Huang, H. Nakamura, J. Med. Chem. 27, 539 (1984)
J.L. Wang, D. Liu, Z.J. Zheng, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z. Huang, Proc. Natl. Acad. Sci. U.S.A. 97, 7124 (2000)
V.K. Ahluwalia, A. Dahiya, V. Garg, Indian J. Biochem. Biophys. 36, 88 (1997)
G.P. Ellis, The chemistry of heterocyclic compounds, in Chromenes, Chromanones, and Chromones, ed. by A. Weissberger, E. C. Taylor (John Wiley, New York, 1977)
D. Heber, C. Heers, U. Ravens, Pharmazie 48, 537 (1993)
W.J. Coates, Chem. Abstr. 113, 40711 (1990)
N. Fokialakis, P. Magiatis, L. Chinou, S. Mitaka, F. Tillequin, Chem. Pharm. Bull. 50, 413 (2002)
P. Beagley, M.A.L. Blackie, K. Chibale, C. Clarkson, R. Meijboom, J.R. Moss, P. Smith, H. Su, Dalton Trans. 15, 3046 (2003)
J.G. Cannon, R.R. Khonji, J. Med. Chem. 18, 110 (1975)
C. Biot, G. Glorian, L.A. Maciejewski, J.S. Brocard, O. Domarle, G. Blampain, G. Blampain, P. Blampain, A.J. Georges, H. Abessolo, D. Dive, J. Lebibi, J. Med. Chem. 40, 3715 (1997)
E.A.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, F.M.A.A. EL-Taweel, Heterocycles 26, 903 (1987)
T.A. Bayer, S. Schafer, H. Breyh, O. Breyhan, C. Wirths, G.A. Treiber, Clin. Neuropathol. 25, 163 (2006)
M.A. Bodaghifard, M. Solimannejad, S. Asadbegi, S. Dolatabadifarahani, Res. Chem. Intermed. 42, 1165 (2016)
S. Banerjee, A. Horn, H. Khatri, G. Sereda, Tetrahedron Lett. 52, 1878 (2011)
Z. Zhou, Y. Zhang, X. Hu, Polycycl. Aromat. Compd. 37, 39 (2017)
K. Niknam, N. Borazjani, R. Rashidian, A. Jamali, Chinese J. Catal. 34, 2245 (2013)
R.S. Bhosale, C.V. Magar, K.S. Solanke, S.B. Mane, S.S. Choudhary, R.P. Pawar, Synth. Commun. 37, 4353 (2007)
F. Mohamadpour, Front. Chem. 10, 934781 (2022)
B. Maleki, S. Sedigh Ashrafi, RSC Adv. 4, 42873 (2014)
M.A. Zolfigol, M. Safaiee, N. Bahrami-Nejad, New J. Chem. 40, 5071 (2016)
S. Banerjee, A. Saha, New J. Chem. 37, 1 (2013)
F. Mohamadpour, Curr. Res. Green Sustain. Chem. 6, 100356 (2023)
B. Maleki, H. Eshghi, M. Barghamadi, N. Nasiri, A. Khojastehnezhad, S. Sedigh Ashrafi, O. Pourshiani, Res. Chem. Intermed. 42, 3071 (2016)
F. Mohamadpour, Polycycl. Aromat. Compd. 41, 160 (2021)
R. Rahnamaf, L. Moradi, M. Khoobi, Res. Chem. Intermed. 46, 2109 (2020)
F. Mohamadpour, Org. Prep. Proced. Int. 54, 306 (2022)
A. Khazaei, F. Gholami, V. Khakyzadeh, A.R. Moosavi-Zare, J. Afsar, RSC Adv. 5, 14305 (2015)
B. Eshtehardian, M. Rouhani, Z. Mirjafary, J. Iran. Chem. Soc. 17, 469 (2020)
S.F. Hojati, N. MoeiniEghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018)
F. Mohamadpour, J. Taiwan Inst. Chem. Eng. 129, 52 (2021)
D. Tahmassebi, J.A. Bryson, S.I. Binz, Synth. Commun. 41, 2701 (2011)
M. Pramanik, K. Choudhuri, A. Mathuri, P. Mal, Chem Comm. 56, 10211 (2020)
H. Kotani, K. Ohkubo, S. Fukuzumi, J. Am. Chem. Soc. 126, 15999 (2004)
Acknowledgements
This work is financially supported by Iran National Science Foundation (INSF) (No. 4015618), financially supported by Iran’s National Elites Foundation (No. 4015618), and also, Shiraz University of Medical Sciences.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
F.M. and H.K. and Sh.Ch. and A.M.A. wrote the main manuscript text and F.M. and H.K. and Sh.Ch. and A.M.A. prepared figures 1-4. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohamadpour, F., Kamyab, H., Chelliapan, S. et al. 9-mesityl-10-methylacridinium perchlorate (Mes-Acr-Me+ClO4−) as a novel metal-free donor–acceptor (D–A) photocatalyst: visible-light-induced access to tetrahydrobenzo[b]pyran scaffolds through a single-electron transfer (SET) pathway. Res Chem Intermed (2024). https://doi.org/10.1007/s11164-024-05304-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11164-024-05304-7