Abstract
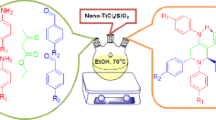
Initially, a new organometallic catalyst with nano-silica gel substrate and Ni active center, namely nano-SiO2@nPr@DPyE-Ni was produced, and its properties were thoroughly debated. It was then applied with excellent performance to produce symmetric di-aryl sulfides (14 entries, 78–98%, 20–150 min, EtOH, and 70 °C), and 2-aryl-5-methyl-2,3-dihydro-1H-3-pyrazolones (12 entries, 91–98%, 2–4 min, solvent-free, and r.t). In both production routes, the nanocatalyst was recyclable and reusable for more than seven runs, and its heterogeneous nature was checked and confirmed through the recycling results, hot filtration test, and ICP-OES (coupled plasma optical emission spectroscopy) method.
Graphical abstract




















Similar content being viewed by others
Availability of data and materials
All data of this work have been reported in the manuscript and supplementary material.
References
V.N. Mahire, G.P. Patil, A.B. Deore, P.G. Chavan, H.D. Jirimali, P.P. Mahulikar, Res. Chem. Intermed. 44, 5801 (2018)
A. Fihri, D. Cha, M. Bouhrara, N. Almana, V. Polshettiwar, Chemsuschem 5, 85 (2012)
R.E. Morsi, R.S. Mohamed, R. Soc, Open Sci. 5, 172021 (2018)
Q. Li, T. Zhou, H. Yang, ACS Catal. 5, 2225 (2015)
L. Wei, S. Yan, H. Wang, H. Yang, NPG Asia Mater. 10, 899 (2018)
M. Rezaei, K. Amani, K. Darvishi, Catal. Commun. 91, 38 (2017)
M.A. Abarghooei, R. Mohebat, Z. Karimi-Jaberi, M.H. Mosslemin, Catal. Commun. 105, 59 (2018)
C. Wang, D. Astruc, Prog. Mater. Sci. 94, 306 (2018)
H. Huang, X. Wang, E. Tervoort, G. Zeng, T. Liu, X. Chen, A. Sologubenko, M. Niederberger, ACS Nano 12, 2753 (2018)
H. Veisi, A. Nikseresht, A. Rostami, S. Hemmati, Res. Chem. Intermed. 45, 507 (2019)
Z. Shahamat, F. Nemati, A. Elhampour, Res. Chem. Intermed. 44, 6649 (2018)
H. Filian, A. Ghorbani-Choghamarani, E. Tahanpesar, J. Iran. Chem. Soc. 16, 2673 (2019)
B. Tahmasbi, A. Ghorbani-Choghamarani, New J. Chem. 43, 14485 (2019)
A. Ghorbani-Choghamarani, Z. Taherinia, M. Mohammadi, Environ. Technol. Innovation. 24, 102050 (2021)
S. Pal, V. Chatare, M. Pal, Curr. Org. Chem. 15, 782 (2011)
H. Filian, A. Kohzadian, M. Mohammadi, A. Ghorbani-Choghamarani, A. Karami, Appl. Organomet. Chem. 34, e5579 (2020)
C.R. Nathaniel, R. Dhanya, P.V. Saranya, G. Anilkumar, ChemistrySelect 7, e202202763 (2022)
C. Parmeggiani, C. Matassini, F. Cardona, Green Chem. 19, 2030 (2017)
A. Yusuf, C. Snape, J. He, H. Xu, C. Liu, M. Zhao, G.Z. Chen, B. Tang, C. Wang, J. Wang, S.N. Behera, Catal. Rev. 59, 189 (2017)
Y. Wang, J. Li, Z. Wei, J. Mater. Chem. A. 6, 8194 (2018)
M.A. Abdelkareem, T. Wilberforce, K. Elsaid, E.T. Sayed, E.A. Abdelghani, A.G. Olabi, Int. J. Hydrogen Energy 46, 23529 (2021)
S.V. Subramaniam, V.K. Dharmalingam, A. Bhattacharya, S. Peruncheralathan, Org. Lett. 25, 8225 (2023)
F.S. Han, Chem. Soc. Rev. 42, 5270 (2013)
L.F. Tietze, A. Modi, Med. Res. Rev. 20, 304 (2000)
F.J. Liu, T.T. Sun, Y.G. Yang, C. Huang, X.B. Chen, RSC adv. 8, 12635 (2018)
B. Jiang, S.J. Tu, P. Kaur, W. Wever, G. Li, J. Am. Chem. Soc. 26, 11660 (2009)
F.A. Pasha, M. Muddassar, M.M. Neaz, S.J. Cho, J. Mol. Graphics Modell. 28, 54 (2009)
D. Castagnolo, F. Manetti, M. Radi, B. Bechi, M. Pagano, A. De Logu, R. Meleddu, M. Saddi, M. Botta, Bioorg. Med. Chem. 17, 5716 (2009)
S. Mor, R. Punia, M. Khatri, D. Kumar, A. Kumar, D.K. Jindal, N. Singh, R. Sharma, M. Ahmed, S. Shukla, K. Jakhar, J. Mol. Struct. 1296, 136759 (2024)
F.A. Ragab, N.M. Abdel-Gawad, H.H. Georgey, M.F. Said, Chem. Pharm. Bull. 61, 834 (2013)
F. Moreau, N. Desroy, J.M. Genevard, V. Vongsouthi, V. Gerusz, G. Le Fralliec, C. Oliveira, S. Floquet, A. Denis, S. Escaich, K. Wolf, M. Busemann, A. Aschenbsenner, Bioorg. Med. Chem. Lett. 18, 4022 (2008)
I. Shaikh, R.N. Jadeja, R. Patel, V. Mevada, V.K. Gupta, J. Mol. Struct. 1232, 130051 (2021)
C.E. Rosiere, M.I. Grossman, Science 113, 651 (1951)
D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. Defelice, M.E. Feigenson, J. Med. Chem. 28, 256 (1985)
Z. Zhao, X. Dai, C. Li, X. Wang, J. Tian, Y. Feng, J. Xie, C. Ma, Z. Nie, P. Fan, M. Qian, Eur. J. Med. Chem. 186, 111893 (2020)
Y. Zhang, K.C. Ngeow, J.Y. Ying, Org. Lett. 9, 3495 (2007)
I.P. Beletskaya, V.P. Ananikov, Chem. Rev. 111, 1596 (2011)
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
M. Lakshman, Journal of Synthetic. Chemistry 1, 148 (2023)
C.T. Barce Ferro, B.F. Dos Santos, C.D.G. da Silva, G. Brand, B.A.L. da Silva, N.L. de Campos Domingues, Curr. Org. Synth. 17, 192 (2020)
L. Rout, T.K. Sen, T. Punniyamurthy, Angew. Chem. Int. Ed. 46, 5583 (2007)
M. Moorthy, A. Govindaraj, B. Madheswaran, B. Kannan, R. Rangappan, ChemistrySelect 1, 4833 (2016)
D. Giuliani, A. Ottani, D. Zaffe, M. Galantucci, F. Strinati, R. Lodi, S. Guarini, Neurobiol. Learn. Mem. 104, 82 (2013)
L. Argueta-Figueroa, O. Martinez-Alvarez, J. Santos-Cruz, R. Garcia-Contreras, L.S. Acosta-Torres, J. De la Fuente-Hernandez, M.C. Arenas-Arrocena, Mater. Sci. Eng. C 76, 1305 (2017)
M. Burkitbayev, N. Bachilova, M. Kurmanbayeva, K. Tolenova, N. Yerezhepova, M. Zhumagul, A. Mamurova, B. Turysbek, G. Demeu, Saudi. J. Biol. Sci. 28, 891 (2021)
M. Nikoorazm, A. Ghorbani-Choghamaranai, M. Khanmoradi, P. Moradi, J. Porous Mater. 25, 1831 (2018)
Y.Q. Almajidi, M. Ubaidullah, B. Pandit, A.K. Kareem, R.M. Romero-Parra, A. Bobirjon, W.R. Kadhum, A.M. Al-Erjan, M. Abosaooda, A.K. Mahmoud, RSC adv. 13, 11393 (2023)
N.A. Barakat, B. Kim, H.Y. Kim, J. Phys. Chem. C 113, 531 (2009)
A. Ghorbani-Choghamarani, Z. Seydyosefi, B. Tahmasbi, Appl. Organomet. Chem. 32, e4396 (2018)
A. Rostami, A. Rostami, M. Ghaderi, S. Gholinejad, Gheisarzadeh. Synthesis. 49, 5025 (2017)
A. Ziarati, J. Safaei-Ghomi, S. Rohani, Ultrason. Sonochem. 20, 1069 (2013)
H. Goudarziafshar, A.R. Moosavi-Zare, Z. Jalilian, M. Abdolmaleki, J. Chin. Chem. Soc. 66, 529 (2019)
Z. Yousofvand, M. Hajjami, F. Ghorbani, R. Ghafouri-Nejad, J. Porous Mater. 25, 1349 (2018)
P. Zhao, H. Yin, H. Gao, C. Xi, J. Org. Chem. 78, 5001 (2013)
A. Ghorbani-Choghamarani, Z. Taherinia, RSC Adv. 6, 59410 (2016)
X. Ku, H. Huang, H. Jiang, H. Liu, J. Comb. Chem. 11, 338 (2009)
P. Gunasekaran, S. Perumal, P. Yogeeswari, D. Sriram, Eur. J. Med. Chem. 46, 4530 (2011)
J. Safaei-Ghomi, E. Afkhami, H. Shahbazi-Alavi, A. Ziarati, Iran. J. Catal. 5, 321 (2015)
Acknowledgments
The authors are grateful to acknowledge the Takin Shimi Sepanta Industries Co, Ilam, Iran.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
H.A synthesized several di-aryl sulfide derivatives (1a–7a) and edited the paper. C.R.B synthesized several pyrazolone derivatives (1b–6b) and designed the graphical abstract. P.B synthesized several di-aryl sulfide derivatives (8a–14a). A.H.R synthesized several di-aryl sulfides derivatives (7b–12b). B.D.O optimized reaction conditions for the preparation of pyrazolone derivatives. A.H.A optimized reaction conditions for the preparation of di-aryl sulfides. M.T.Q characterized the skeleton of the catalyst via HRTEM, FESEM, and XRD analyses. A.K characterized the skeleton of the catalyst by ICP, EDS, and elemental mapping analyses. Y.F.M characterized the skeleton of the catalyst through TGA, FTIR, and BET analyses. M.S.G.N fabricated the catalyst and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AlMohamadi, H., Rodriguez-Benites, C., Bansal, P. et al. Nano-SiO2@nPr@DPyE-Ni: a novel nanocatalyst for the rapid production of symmetric di-aryl sulfides and pyrazolones. Res Chem Intermed (2024). https://doi.org/10.1007/s11164-024-05296-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11164-024-05296-4