Abstract
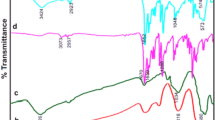
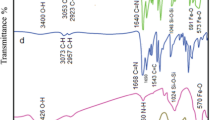
In this work a new core–shell nanocatalyst, Fe3O4@SiO2/Schiff base of Ni(II), (Fe3O4@SiO2/SB(Ni)), was synthesized and characterized. The Schiff base immobilized on magnetite nanoparticles introduced in this study, is a simple and aliphatic Schiff base. The synthesis steps were meticulously followed. First, magnetite-silica nanoparticles were prepared and functionalized by –NH2 groups using 3-amino propyl triethoxysilane (APTES). Subsequently, the aliphatic Schiff base ligand was immobilized through a condensation reaction between the –NH2 groups on the magnetite-silica and the –C=O groups of acetylacetone. The supported Schiff base complex of Ni(II) on magnetite-silica was synthesized by a reaction between immobilized Schiff base and Ni(II) acetate tetrahydrate salt. Finally, the immobilized Ni(II) Schiff base complex was fully characterized using several techniques including, FT-IR, VSM, XRD, FE-SEM, EDX, TEM, BET, TGA-DTA, and AAS. The magnetically recoverable core–shell nanocatalyst, demonstrated remarkable catalytic activity in the synthesis of 3,4-dihydropyrimidine-2-(1H)-one. This reaction involved three components: an aldehyde, β-keto ester, and urea, and was carried out via the solvent-free Biginelli reaction. The results indicate that the products are synthesized in good to excellent yields (82–91%) within 13–22 min. At the end of the reaction, the nanocatalyst can be easily removed from the reaction mixture using an external magnet and reused several times. The synthesized products were purified and characterized by FT-IR and 1H NMR techniques.
Graphical abstract
















Similar content being viewed by others
Availability of data and materials
We declare that the all research data related to the article is available in the text of the article and supplementary file.
References
H. Nagarajaiah, A. Mukhopadhyay, J.N. Moorthy, Tetrahedron Lett. 57, 5135 (2016)
R.R. Alavala, U. Kulandaivelu, P. Bonagiri, S. Boyapati, V. Jayaprakash, A.T. Subramaniam, Anti-Infec. Agents 13, 154 (2015)
A.E. Huseynzada, C. Jelch, H.V.N. Akhundzada, S. Soudani, C.B. Nasr, A. Israyilova, F. Doria, U.A. Hasanova, R.F. Khankishiyeva, M. Freccero, RSC Adv. 11, 6312 (2021)
G. Lauro, M. Strocchia, S. Terracciano, I. Bruno, K. Fischer, C. Pergola, O. Werz, R. Riccio, G. Bifulco, Eur. J. Med. Chem. 80, 407 (2014)
M.H. El-Wakil, M. Teleb, M.M. Abu-Serie, S. Huang, G.W. Zamponi, H. Fahmy, Bioorg. Chem. 115, 105262 (2021)
H.M. Marvaniya, P.K. Parikh, D.J. Sen, J. Appl. Pharm. Sci. 1, 109 (2011)
H. Yuan, K. Zhang, J. Xia, X. Hu, S. Yuan, Cogent Chem. 3, 1318692 (2017)
T. Sekhar, P. Thriveni, M. Harikrishna, K. Murali, Asian J. Chem. 30, 1243 (2018)
M. Afradi, N. Foroughifar, H. Pasdar, H. Moghanian, RSC Adv. 6, 59343 (2016)
E. Abbaspour-Gilandeh, S.C. Azimi, A. Mohammadi-Barkchai, RSC Adv. 4, 54854 (2014)
J. Safari, S. Gandomi-Ravandi, J. Mol. Struct. 1074, 71 (2014)
L.-Q. Kang, D.-Y. Jin, Y.-Q. Cai, Synth. Commun. 43, 1896 (2013)
S. Bentahar, M.A. Taleb, A. Sabour, A. Dbik, M. El Khomri, N. El Messaoudi, A. Lacherai, R. Mamouni, Russ. J. Org. Chem. 55, 1423 (2019)
M. Kamali, Int. J. Chem. Tech. Res. 8, 536 (2015)
M. Norouzi, N. Noormoradi, M. Mohammadi, Nanoscale Adv. 5, 6594 (2023)
M. Kazemi, M. Mohammadi, Appl. Organomet. Chem. 34, e5400 (2020)
M. Mohammadi, A. Ghorbani-Choghamarani, S.M. Ramish, J. Mol. Struct. 1292, 136115 (2023)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour, H. Zeynali, Inorg. Chem. 61, 4825 (2022)
M. Zhu, G. Diao, J. Phys. Chem. C 115, 24743 (2011)
M. Sheykhan, A. Yahyazadeh, L. Ramezani, Mol. Catal. 435, 166 (2017)
S. Akbarpour, A. Bezaatpour, E. Askarizadeh, M. Amiri, Appl. Organomet. Chem. 31, e3804 (2017)
A. Bezaatpour, S. Khatami, M. Amiri, RSC Adv. 6, 27452 (2016)
M. Hajjami, F. Sharifirad, F. Gholamian, Appl. Organomet. Chem. 31, e3844 (2017)
A.H. Lu, E.L. Salabas, F. Schüth, Angew. Chem. Int. Ed. Engl. 46, 1222 (2007)
M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami, A. Ghorbani-Choghamarani, J. Ind. Eng. Chem. 97, 1 (2021)
F. Ghobakhloo, M. Mohammadi, M. Ghaemi, D. Azarifar, ACS Appl. Nano Mater. 7, 1265 (2024)
A. Spoială, C.-I. Ilie, L.N. Crăciun, D. Ficai, A. Ficai, E. Andronescu, Appl. Sci. 11, 11075 (2021)
M. Mohammadi, A. Ghorbani-Choghamarani, Res. Chem. Intermed. 48, 2641 (2022)
H. Keypour, J. Kouhdareh, S. Alavinia, K. Rabiei, M. Mohammadi, A. Maryamabadi, S. Babaei, J. Organomet. Chem. 989, 122646 (2023)
M. Mohammadi, A. Ghorbani-Choghamarani, RSC Adv. 12, 26023 (2022)
A.M. Abu-Dief, I.M. Mohamed, Beni-Suef Univ. J. Basic Appl. Sci. 4, 119 (2015)
Z. Asadi, M. Asadi, M. Setoodehkhah, Spectrochim. Acta A Mol. Biomol. 112, 214 (2013)
M. Asadi, M.S. Khah, J. Iran. Chem. Soc. 7, 875 (2010)
K. Mohammadi, M. Asadi, M.S. Khah, H. Sepehrpour, Croat. Chem. Acta (2016). https://doi.org/10.5562/cca2706
K. Mohammadi, M. Asadi, M. Setoodeh Khah, H. Sepehrpour, Croat. Chem. Acta 89, 277 (2016)
B.S. Lane, K. Burgess, Chem. Rev. 103, 2457 (2003)
J.N. Pédeutour, K. Radhakrishnan, H. Cramail, A. Deffieux, Macromol. Rapid Commun. 22, 1095 (2001)
P. Manisankar, A. Gomathi, D. Velayutham, J. Solid State Electrochem. 9, 601 (2005)
A. Ríos-Escudero, M. Villagrán, F. Caruso, J. Muena, E. Spodine, D. Venegas-Yazigi, L. Massa, L. Todaro, J. Zagal, G. Cárdenas-Jirón, Inorganica Chim. Acta 359, 3947 (2006)
C. Heinrichs, W.F. Hölderich, Catal. Lett. 58, 75 (1999)
R.E. Malekshah, B. Fahimirad, A. Khaleghian, Int. J. Nanomed. 15, 2583 (2020)
M. Setoodehkhah, A. Mazraati, M. Moradian, J. Inorg. Organomet. Polym., (2021)
A.R. Kiasat, J. Davarpanah, Res. Chem. Intermed. 41, 2991 (2015)
R. Gurav, A. Gurav, S. Salunkhe-Gawali, S. Jadhav, P. Choudhari, S. Sankpal, S. Hangirgekar, Appl. Organomet. Chem. 36, e6547 (2022)
A. Mobinikhaledi, N. Foroughifar, A. Khajeh-Amiri, React. Kinet. Mech. 117, 59 (2016)
L.V. Chopda, P.N. Dave, ChemistrySelect 5, 5552 (2020)
A. Mazraati, M. Setoodehkhah, M. Moradian, J. Inorg. Organomet. Polym. Mater. 32, 143 (2022)
Z. Karimi-Jaberi, M.S. Moaddeli, M. Setoodehkhah, M.R. Nazarifar, Res. Chem. Intermed. 42, 4641 (2016)
M. Ghanbari, S. Moradi, M. Setoodehkhah, Green Chem. Lett. Rev. 11, 111 (2018)
S. Yazdanseta, K. Yasin, M. Setoodehkhah, M. Ghanbari, E. Fadaee, Res. Chem. Intermed. 48, 1 (2022)
A. Mazraati, M. Setoodehkhah, M. Moradian, J. Cluster Sci. 35, 1 (2024)
F. Nemati, M.M. Heravi, R.S. Rad, Chin. J. Catal. 33, 1825 (2012)
M. Sonmez, M. Georgescu, L. Alexandrescu, D. Gurau, A. Ficai, D. Ficai, E. Andronescu, Curr. Pharm. Des. 21, 5324 (2015)
T. Karimpour, E. Safaei, B. Karimi, Y.I. Lee, ChemCatChem 10, 1889 (2018)
A. Noormohamadi, M. Homayoonfal, M.R. Mehrnia, F. Davar, Ceram. Int. 43, 17174 (2017)
W.F. Elmobarak, F. Almomani, Chemosphere 265, 129054 (2021)
Z. Zhang, W. Zhang, in 2015 International Symposium on Energy Science and Chemical Engineering. (Atlantis Press, 2015), pp. 180.
W. Wang, P. Liu, M. Zhang, J. Hu, F. Xing, Open J. Compos. Mater. 2, 104 (2012)
Z. Ramazani, D. Elhamifar, M. Norouzi, R. Mirbagheri, Compos. B Eng. 164, 10 (2019)
R. Velpula, J. Banothu, R. Gali, R. Deshineni, R. Bavantula, Chin. Chem. Lett. 26, 309 (2015)
Z. Liu, R. Ma, D. Cao, C. Liu, Molecules 21, 462 (2016)
P. Wu, L. Feng, Y. Liang, X. Zhang, B. Mahmoudi, M. Kazemnejadi, Appl. Catal. A-Gen. 590, 117301 (2020)
Acknowledgements
The authors are deeply grateful to the University of Kashan for financial support of this research project.
Funding
All of the sources of funding for the work described in this publication are acknowledged below: University of Kashan (IRAN) was financial support of this research project.
Author information
Authors and Affiliations
Contributions
We confirm that the manuscript has been read and approved by all named authors and the order of authors listed in the manuscript has been approved by all named authors.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical approval
This declaration is “not applicable”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abravi, Z., Setoodehkhah, M. & Moradian, M. Synthesis and characterization of Ni(II) complex supported on magnetite-silica nanoparticles and investigation of its catalytic activity in Biginelli reaction under solvent-free conditions. Res Chem Intermed 50, 2067–2090 (2024). https://doi.org/10.1007/s11164-024-05273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05273-x