Abstract
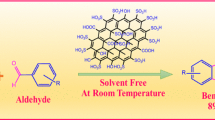
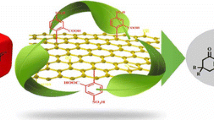
A simple and straightforward synthetic strategy has been established toward furnishing of promising bis-heterocyclic pyrazole containing substituted tetrazole derivatives which might be proved as valuable molecules because of the presence of medicinally and biologically important pyrazole and tetrazole entities. Sulfonic acid decorated graphene oxide (GO-SO3H), a new class of heterogeneous carbo-catalyst was first time reported for synthesis of tert-butyl/ethyl acetate and pyrazole-substituted tetrazole through one-pot 4-C reaction. The synthesized GO-SO3H catalyst offered more than 95% yields of tetrazoles with a broad range of substrates including different substituted aldehyde, isocyanide, and amine within 15–20 min under mild reaction conditions. In addition, sulfonic acid decorated graphene oxide (GO-SO3H) was reused up to five reaction cycles without considerable drop in its catalytic efficiency. The formation of all synthesized pyrazole containing substituted tetrazole derivatives was confirmed by various spectroscopic techniques.









Similar content being viewed by others
Data availability
All datasets used can be accessed through supporting information.
References
Z. Ke, B. Yu, H. Wang, J. Xiang, J. Han, Y. Wu, Z. Liu, P. Yang, Z. Liu, Green Chem. 21, 1695 (2019)
G.-F. Qin, Q.-Y. Qin, B.-F. Long, D.-P. Wei, Y.-H. Xu, S.-J. Bao, X.-H. Yin, J. Iran. Chem. Soc. 14, 1227 (2017)
B. Atashkar, M.A. Zolfigol, S. Mallakpour, Mol. Catal. 452, 192 (2018)
S.D. Naik, K. Hosamani, S.K. Vootla, Chem. Data Collect. 15, 207 (2018)
Y. Sun, L. Zheng, Y. Yang, X. Qian, T. Fu, X. Li, Z. Yang, H. Yan, C. Cui, W. Tan, Nano-Micro Lett. 12, 1 (2020)
C.G. Neochoritis, T. Zhao, A. Dömling, Chem. Rev. 119, 1970 (2019)
E.A. Popova, R.E. Trifonov, V.A. Ostrovskii, Russ. Chem. Rev. 88, 644 (2019)
S.S. Karbasakia, G. Bagherzadea, B. Malekib, M. Ghanic, J. Taiwan Inst. Chem. Eng. 118, 342 (2021)
M.A. Malik, S.A. Al-Thabaiti, M.A. Malik, Int. J. Mol. Sci. 13, 10880 (2012)
D. Varadaraji, S.S. Suban, V.R. Ramasamy, K. Kubendiran, J.S.K.G. Raguraman, S.K. Nalilu, H.N. Pati, Org. Commun. 3, 45 (2010)
V.A. Ostrovskii, R.E. Trifonov, E.A. Popova, Russ. Chem. Bull. 61, 768 (2012)
D. Fischer, T.M. Klapötke, J. Stierstorfer, Angew. Chem. Int. Ed. 54, 10299 (2015)
B.S. Jursic, B.W. Leblanc, J. Heterocycl. Chem. 35, 405 (1998)
G. Sandmann, C. Schneider, P. Böger, Z. Naturforsch.ung C. 51, 534 (1996)
S.D. Guggilapu, S.K. Prajapti, A. Nagarsenkar, K.K. Gupta, B.N. Babu, Synlett 27, 1241 (2016)
S. Swami, N. Devi, A. Agarwala, V. Singh, R. Shrivastava, Tetrahedron Lett. 57, 1346 (2016)
R. Taghavi, S. Rostamnia, Chem. Methodol. 6, 639 (2022)
A.Z. Hattab, N. Al-Lami, J.S. Wadi, Eurasian Chem. Commun. 4, 222 (2022)
H. Alinezhad, M. Tajbakhsh, B. Maleki, F.P. Oushibi, Polycycl. Aromat. Compd. 40, 1485 (2019)
A.R. Moosavi-Zare, H. Goudarziafshar, Z. Jalilian, F. Hosseinabadi, Chem. Methodol. 6, 571 (2022)
S.S. Karbasakia, G. Bagherzadea, B. Malekib, M. Ghani, Org. Prep. Proced. Int. 53, 498 (2021)
M. Halder, M.M. Islam, P. Singh, A.S. Roy, S.M. Islam, K. Sen, ACS Omega 3, 8169 (2018)
P. Moradi, A. Ghorbani-Choghamarani, Appl. Organomet. Chem. 31, e3602 (2017)
P.K. Samanta, R. Biswas, T. Das, M. Nandi, B. Adhikary, R.M. Richards, P. Biswas, J. Porous Mater. 26, 145 (2019)
S. Molaei, T. Tamoradi, M. Ghadermazi, A.G. Choghamarani, Micropor. Mesopor. Mater. 272, 241 (2018)
B. Salahshournia, H. Hamadi, V. Nobakht, Appl. Organomet. Chem. 32, e4416 (2018)
J.A. Mehraban, K. Azizi, M.S. Jalali, A. Heydari Chem. Select. 3, 116 (2018)
M. Koolivand, M. Nikoorazm, A.G. Choghamarani, M. Mohammadi, Appl. Organomet. Chem. 36, e6656 (2022)
M. Sajjadi, M. Nasrollahzadeh, H. Ghafuri, T. Baran, Y. Orooji, N.Y. Baran, M. Shokouhimehr, Int. J. Biol. Macromol. 209, 1573 (2022)
M.S. Mashhoori, R. Sandaroos, Sci. Rep. 12, 15364 (2022)
M.B. Swami, A.H. Jadhav, S.R. Mathpati, H.G. Ghuge, S.G. Patil, Res. Chem. Intermed. 43, 2033 (2017)
M.M. Aghayan, M. Alizadeh, M.M. Tavana, R. Boukherroub, Tetrahedron Lett. 55, 6694 (2014)
P. Basak, S. Dey, P. Ghosh, Chem. Select. 5, 626 (2020)
S. Keshipour, S. Mohammad-Alizadeh, Sci. Rep. 11, 16148 (2021)
S. Keshipour, S. Mohammad-Alizadeh, M.H. Razeghi, J. Phys. Chem. Solids. 161, 110434 (2022)
A. Al-Azmi, S. Keshipour, J. Colloid Interface. Sci. 612, 701 (2022)
S.M. Salehi, S. Keshipour, F. Ahour, J. Phys. Chem. Solids. 176, 111239 (2023)
S. Keshipour, A. Al-Azmi, Appl. Organomet. Chem. 34, e5311 (2020)
H. Alinezhad, M. Tarahomi, B. Maleki, A. Amiri, Appl. Organomet. Chem. 33, e4661 (2019)
H.P. Jia, D.R. Dreyer, C.W. Bielawski, Adv. Synth. Catal. 353, 528 (2011)
I.K. Basha, E.M. Abd El-Monaem, R.E. Khalifa, A.M. Omer, A.S. Eltaweil, Sci. Rep. 12, 9339 (2022)
A.H. Jadhav, K. Lee, S. Koo, J.G. Seo, RSC Adv. 5, 26197 (2015)
P. Pattanayak, F. Papiya, N. Pramanik, P.P. Kundu, Sustain. Energy Fuels. 3, 1808 (2019)
R.A. Dar, G.A. Naikoo, A.K. Srivastava, IUl. Hassan, S.P. Karna, L. Giri, A.M.H. Shaikh, M. Rezakazemi, W. Ahmed, Sci. Rep. 12, 117 (2022)
J. Liu, Y. Xue, L. Dai, J. Phy. Chem. Lett. 3, 1928 (2012)
S. Swami, A. Agarwala, R. Shrivastava, Mol. Divers. 21, 81 (2017)
A.R. Kazemizadeh, N. Hajaliakbari, R. Hajian, N. Shajari, A. Ramazani, Hel. Chim. Acta. 95, 594 (2012)
M.N.S. Rad, S. Behrouz, V.S. Dehchenari, S.J. Hoseini, J. Heterocycl. Chem. 54, 355 (2017)
M.L. Kantam, K.S. Kumar, C. Sridhar, Adv. Synth. Catal. 347, 1212 (2005)
T. Tamoradi, A.G. Choghamarani, M. Ghadermazi, New J. Chem. 41, 11714 (2017)
P. Akbarzadeh, N. Koukabi, M.M. Hosseini, J. Heterocycl. Chem. 57, 2455 (2020)
H. Naeimi, S. Mohamadabadi, Dalton Trans. 43, 12967 (2014)
G. Qi, W. Zhang, Y. Dai, Res. Chem. Intermed. 41, 1149 (2015)
M. Nasrollahzadeh, B. Jaleh, A. Jabbari, Rsc Adv. 4, 36713 (2014)
A.F. Hegarty, N.M. Tynan, S. Fergus, J. Chem. Soc. Perkin Trans. 2(7), 1328 (2002)
Acknowledgements
N Sand R S are thankful to the Manipal University Jaipur, Department of Chemistry for resources infrastructures and money grant. The author also would like to thank the Central Analytical Facilities (CAF) and Sophisticated Analytical Instrument Facility (SAIF) laboratory of Manipal University Jaipur SEM, XRD, EDX, FT-IR, and NMR spectra analysis reported in this paper.
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by NS, SS, and SP. The first draft of the manuscript was written by NS and SS and corrected by RS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The authors declare that the research process did not involve any human or animal experiments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, N., Swami, S., Pathak, S. et al. Sulfonic acid decorated graphene oxide (GO-SO3H): a efficient heterogeneous catalyst for synthesis of tert-butyl/ethylacetate and pyrazole disubstituted tetrazole derivatives. Res Chem Intermed 49, 3441–3459 (2023). https://doi.org/10.1007/s11164-023-05047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05047-x