Abstract
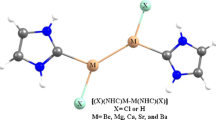
During this study, six carbenes, consisted of various substitutions (hydrogen, deuterium, fluorine, chlorine, bromine, and methyl) and their 24 related carbenoids (using Li, Na, Be, and Mg metals) were designed. These species were theoretically studied to obtain the extensive and comprehensive information about their structures, stabilities, atomic specifications, and bonding properties using MP2/aug-cc-pVTZ level of theory. Moreover, the PBE1PBE DFT method was used for IQA analyses using AIMAll program package. The calculated molecular parameters showed that the electronegativity and the size of ligand are effective on the studied structures. Moreover, the electronegativity effect is more important than the size. Atomic hybridizations results showed the p indexes of carbon in triplet carbenes are also smaller than those in singlet carbenes, but this difference in halogen-containing carbenes is smaller than the other carbenes. In population analyses, except for sodium-based carbenoids, all carbenoids have higher Eg values than the carbenes. The ΔG values for α-elimination reaction, as a method of preparation of these carbenes, were obtained in order of f < c < b < m < h, which is reversely related to the electronegativities of the connected ligands. IQA analyses were performed to evaluate the relative stability of carbenes. It was found that the classical interaction in C–F is attractive (negative) unlike the other mentioned bonds energy for carbenes. This electrostatic term in C–F is larger in the singlet state than the triplet state, which leads to the singlet state of CF2 being more stable and consequently more favorable than its triplet state.





Similar content being viewed by others
Availability of data and materials
All data are available and could be sent on request from the corresponding author.
References
R. A. Moss, M. S. Platz, M. Jones Jr, (Eds.). John Wiley & Sons (2004)
G.T. Gurmessa, G.S. Singh, Res. Chem. Intermed. 43, 6447 (2017)
F.E. Hahn, Chem. Rev. 118(19), 9455 (2018)
M.S. Lee, J.E. Jackson, Res. Chem. Intermed. 20, 223 (1994)
J. Vignolle, X. Cattoen, D. Bourissou, Chem. Rev. 109(8), 3333 (2009)
H. Tomioka, E. Iwamoto, H. Itakura, K. Hirai, Nature 412(6847), 626 (2001)
A. Pawar, S. Gajare, A. Patil, R. Kurane, G. Rashinkar, S. Patil, Res. Chem. Intermed. 47, 2801 (2021)
J.C. Lin, R.T. Huang, C.S. Lee, A. Bhattacharyya, W.S. Hwang, I.J. Lin, Chem. Rev. 109(8), 3561 (2009)
J. J. Zupancic, G. B. Schuster J. Am. Chem. Soc. 102(18), 5958 (1980).
D. Bourissou, O. Guerret, F.P. Gabbaï, G. Bertrand, Chem. Rev. 100(1), 39 (2000)
P.B. Grasse, B.E. Brauer, J.J. Zupancic, K.J. Kaufmann, G.B. Schuster, J. Am. Chem. Soc. 105(23), 6833 (1983)
A. Nemirowski, P.R. Schreiner, J. Org. Chem. 72(25), 9533 (2007)
D.R. Myers, V. P. Senthilnathan, M. S. Platz, M. Jones, J. Am. Chem. Soc. 108(14) 4232 (1986)
G. Bertrand, Carbene chemistry: from fleeting intermediates to powerful reagents. Boca Raton: CRC Press (2002).
P.H. Mueller et al., J. Am. Chem. Soc. 103(17), 5049 (1981)
K. Hirai, T. Itoh, H. Tomioka, Chem. Rev. 109(8), 3275 (2009)
C. Boehme, G. Frenking, J. Am. Chem. Soc. 118(8), 2039 (1996)
Y. Mizuhata, T. Sasamori, N. Tokitoh, Chem. Rev. 109(8), 3479 (2009)
A.J. Arduengo, Acc. Chem. Res. 32(11), 913 (1999)
C. Hill, F. Bosold, K. Harms, J.C. Lohrenz, M. Marsch, M. Schmieczek, G. Boche, Chem. Ber. 130(9), 1201 (1997)
P. Bazinet, G.P. Yap, D.S. Richeson, J. Am. Chem. Soc. 125(44), 13314 (2003)
A.J. Arduengo III., H.R. Dias, D.A. Dixon, R.L. Harlow, W.T. Klooster, T.F. Koetzle, J. Am. Chem. Soc. 116(15), 6812 (1994)
P.H. Mueller, N.G. Rondan, K.N. Houk, J.F. Harrison, D. Hooper, B.H. Willen, J.F. Liebman, J. Am. Chem. Soc. 103(17), 5049 (1981)
E.A. Carter, W.A. Goddard, J. Phys. Chem. 91(18), 4651 (1987)
F. Mendez, M.A. Garcia-Garibay, J. Org. Chem. 64(19), 7061 (1999)
C.M. Geise, Y. Wang, O. Mykhaylova, B.T. Frink, J.P. Toscano, C.M. Hadad, J. Org. Chem. 67(9), 3079 (2002)
M. Soleilhavoup, G. Bertrand, Acc. Chem. Res. 48(2), 256 (2015)
D. Munz, Organometallics 37(3), 275 (2018)
M.S. Sanford, M. Ulman, R.H. Grubbs, J. Am. Chem. Soc. 123(4), 749 (2001)
A. Caballero, P.J. Pérez, Chem. Eur. J. 23(58), 14389 (2017)
L. Friedman, H. Shechter, J. Am. Chem. Soc. 82(4), 1002 (1960)
P.S. Skell, A.Y. Garner, J. Am. Chem. Soc. 78(20), 5430 (1956)
R.A. Moss, Acc. Chem. Res. 13(2), 58 (1980)
A. Padwa, M.D. Weingarten, Chem. Rev. 96(1), 223 (1996)
T. Satoh, Chem. Soc. Rev. 36(10), 1561 (2007)
G. Costantino, R. Rovito, A. Macchiarulo, R. Pellicciari, THEOCHEM 581(1–3), 111 (2002)
L.R. Domingo, M. Ríos-Gutiérrez, M. Duque-Noreña, E. Chamorro, P. Pérez, Theor. Chem. Acc. 135(7), 1 (2016)
J. Messelberger, A. Grünwald, P. Pinter, M.M. Hansmann, D. Munz, Chem. Sci. 9(28), 6107 (2018)
J. Vaitla, A. Bayer, K.H. Hopmann, Angew. Chem. Int. Ed. 57(49), 16180 (2018)
H. Qiu, C. Deng, Chem. Phys. Lett. 249(3–4), 279 (1996)
H. Hermann, J.C. Lohrenz, A. Kühn, G. Boche, Tetrahedron 56(25), 4109 (2000)
T. Clark, P. V. R. Schleyer, J. Chem. Soc. Chem. Comm. (20), 883 (1979)
M.A. Vincent, H.F. Schaefer, J. Chem. Phys. 77(12), 6103 (1982)
T. Clark, P.V.R. Schleyer, Tetrahedron Lett. 20(51), 4963 (1979)
B.T. Luke, J.A. Pople, P.V.R. Schleyer, T. Clark, Chem. Phys. Lett. 102(2–3), 148 (1983)
T. Clark, P.V.R. Schleyer, J. Am. Chem. Soc. 101(26), 7747 (1979)
T. Koizumi, O. Kikuchi, Bull. Chem. Soc. Japan 68(1), 120 (1995)
R.H. Nishimura, V.E. Murie, R.A. Soldi, J.L. Lopes, G.C. Clososki, J. Braz.Chem. Soc. 26, 2175 (2015)
K.G. Taylor, Tetrahedron 38(18), 2751 (1982)
S. Molitor, V.H. Gessner, Angew. Chem. Int. Ed. 55(27), 7712 (2016)
V.H. Gessner, Chem. Comm. 52(81), 12011 (2016)
Gaussian 09, Revision B.01, M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A.V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H.P. Hratchian, J.V. Ortiz, A.F. Izmaylov, J.L. Sonnenberg, D. Williams-Young, F.Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V.G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, V. N., T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J.B. Foresman, D. J. Fox, Gaussian, Inc., Wallingford CT, (2016).
AIMAll (Version 19.10.12), Todd A. Keith, TK Gristmill Software, Overland Park KS, USA, 2019 (aim.tkgristmill.com)
V. GaussView, 6, Dennington, Roy (Todd A.; Millam, John M. Semichem Inc., Shawnee Mission, KS, Keith, 2016)
T. Koopmans, Physica 1, 104 (1933)
R.G. Parr, R.G. Pearson, J. Am. Chem. Soc. 105(26), 7512 (1983)
A.M. Pendás, M. Blanco, E. Francisco. J. Chem. Phys. 120, 4581 (2004)
M. Menéndez, R. Álvarez Boto, E. Francisco, Á. Martín Pendás J. Comput. Chem. 36, 833 (2015)
M. A. Blanco, A. Martín Pendás, E. Francisco, J. Chem. Theory Comput. 1(6), 1096 (2005)
A. Martín Pendás, M. Blanco, E. Francisco, J. Chem. Phys. 125, 184112 (2006).
J.L. Casals-Sainz, F. Jiménez-Grávalos, A. Costales, E. Francisco, Á.M. Pendás, J. Phys. Chem. A 122, 849 (2018)
Funding
No fund is received for this work.
Author information
Authors and Affiliations
Contributions
ZE performed MP2 and DFT calculations and extracted the related data. HT designed the study, analyzed MP2 and DFT data, prepared related figures, tables, and scheme, and wrote the all parts of the manuscript except IQA part. KS performed IQA calculations and wrote related part in the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no financial or personal interest.
Ethical approval
This work does not include any human and/or animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emami-Meibodi, Z., Tavakol, H. & Eskandari, K. MP2, DFT, and IQA study of substituent effect on the structure, stability, and bonding properties of CX2 singlet and triplet carbenes and related carbenoids. Res Chem Intermed 49, 3205–3225 (2023). https://doi.org/10.1007/s11164-023-05031-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05031-5