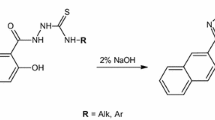
Abstract
New thio/carbohydrazone derivatives (1–10) have been synthesized from various iso(thio)/cyanates. The chemical structures of synthesized compounds were elucidated with UV–Vis, IR, 1H NMR, 13C NMR spectroscopic methods, and elemental analysis. Antimicrobial activities of all synthesized compounds against Gram-positive, Gram-negative mold and yeast were screened by disc diffusion and microdilution methods. Ground state structures were obtained with the DFT approach, and also, experimental data were supported by spectroscopy calculations. In addition to calculating the reactivity parameters, intramolecular interactions and electron density distributions were analyzed and approaches to the antimicrobial properties of the compounds were presented. Furthermore, the interaction between the compounds and pBR322 plasmid DNA was investigated by gel electrophoresis. In this study to investigate the antibacterial and antifungal activity of new thio/carbohydrazone derivatives (1–10), it was determined that compound 7 has a remarkable inhibitory effect on S. aureus (12.66 ± 1.52 mm) and compound 10 on S. aureus (20.33 ± 0.57 mm), S. mutans (16.33 ± 0.57 mm) and A. niger (15.33 ± 0.57 mm). The interaction results of compounds 1–10 with plasmid pBR322 DNA showed that compounds 1, 8, and 10 caused a reduction in the densities of form I and form II DNA. Compounds 2–7, 9 caused a double-stranded break of plasmid DNA (Form III).
Graphical abstract
New thio/carbohydrazone derivatives have been synthesized. Structures of all compounds have been elucidated with spectroscopic approaches. Antimicrobial activities of all synthesized compounds were determined. The interaction between the compounds and pBR322 plasmid DNA was investigated by gel electrophoresis. DFT studies were performed about reactivity parameters, intramolecular interactions and electron density distributions.








Similar content being viewed by others
Data availability
The results/data/figures in this manuscript have not been published elsewhere, nor are they under consideration by another publisher.
References
M.S. Çavuş, H. Yakan, C. Özorak, H. Muğlu, T.K. Bakır, Res. Chem. Intermed. 48, 1593 (2022)
H. Muğlu, B.Z. Kurt, F. Sönmez, E. Güzel, M.S. Çavuş, H. Yakan, J. Phys. Chem. Solids 164, 110618 (2022)
Z.H. Chohan, H. Pervez, K.M. Khan, C.T. Supuran, J. Enzyme Inhib. Med. Chem. 20, 81 (2005)
G.B. Bagihalli, P.G. Avaji, P.S. Badami, S.A. Patil, J. Coord. Chem. 61, 2793 (2008)
K. El-Mahdy, A. El-Kazak, M. Abdel-Megid, M. Seada, O. Farouk, Acta Chim. Slov. 63, 18 (2016)
A.R. Božić, S.K. Bjelogrlić, I.T. Novaković, N.R. Filipović, P.M. Petrović, A.D. Marinković, T.R. Todorović, I.N. Cvijetić, ChemistrySelect 3, 2215 (2018)
M.T. Muhammad, N. Ghouri, K.M. Khan, M.I. Choudhary, S. Perveen, Med. Chem. 14, 725 (2018)
K. Gangarapu, S. Manda, A. Jallapally, S. Thota, S.S. Karki, J. Balzarini, E. De Clercq, H. Tokuda, Med. Chem. Res. 23, 1046 (2014)
M.T. Gabr, N.S. El-Gohary, E.R. El-Bendary, N. Ni, M.I. Shaaban, M.M. El-Kerdawy, Synth. Commun. 48, 2899 (2018)
M. Sathisha, V. Revankar, K. Pai, Met. Based Drugs 2008 (2008)
K. Fink, M. Uchman, Coord. Chem. Rev. 431, 213684 (2021)
G. Elmacı, H. Duyar, B. Aydıner, I. Yahaya, N. Seferoğlu, E. Şahin, S.P. Çelik, L. Açık, Z. Seferoğlu, J. Mol. Struct. 1184, 271 (2019)
A. Bolhuis, J.R. Aldrich-Wright, Bioorg. Chem. 55, 51 (2014)
M. Kumar, V. Kumar, V. Beniwal, Med. Chem. Res. 24, 2862 (2015)
A. Okumuş, G. Elmas, A. Binici, B. Aydın, L. Açık, Z. Kılıç, T. Hökelek, Inorg. Chim. Acta 538, 121001 (2022)
A.M. Abu-Dief, R.M. El-Khatib, F.S. Aljohani, H.A. Al-Abdulkarim, S. Alzahrani, G. El-Sarrag, M. Ismael, Comput. Biol. Chem. 97, 107643 (2022)
I.J. Simpson, M. Lee, A. Kumar, D.W. Boykin, S. Neidle, Bioorg. Med. Chem. Lett. 10, 2593 (2000)
M. Chemchem, I. Yahaya, B. Aydıner, N. Seferoğlu, O. Doluca, N. Merabet, Z. Seferoğlu, Tetrahedron 74, 6897 (2018)
R. Mehandi, R. Arif, M. Rana, S. Ahmedi, R. Sultana, M.S. Khan, M. Maseet, M. Khanuja, N. Manzoor, N. Nishat, J. Mol. Struct. 1245, 131248 (2021)
P. Mondal, P. Sengupta, U. Pal, S. Saha, A. Bose, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 245, 118936 (2021)
P. Hohenberg, W. Kohn, Phys. Rev. 136, B864 (1964)
W. Kohn, L.J. Sham, Phys. Rev. 140, A1133 (1965)
M.J. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. Petersson et al., Gaussian 09 (Revision A.02) (Gaussian Inc., Wallingford, 2009)
T. Lu, F. Chen, J. Comput. Chem. 33, 580 (2012)
R.F. Bader, Acc. Chem. Res. 18, 9 (1985)
R.F. Bader, Chem. Rev. 91, 893 (1991)
T. Lu, Q. Chen, Chem. Methods 1, 231 (2021)
C. Clsi, Clin. Lab Stand. Inst. 35, 16 (2016)
Ö. Ertürk, Biologia 61, 275 (2006)
H. Akbaş, A. Okumuş, Z. Kılıç, T. Hökelek, Y. Süzen, L.Y. Koç, L. Açık, Z.B. Çelik, Eur. J. Med. Chem. 70, 294 (2013)
N. Asmafiliz, Z. Kılıç, A. Öztürk, Y. Süzen, T. Hökelek, L. Açık, Z.B. Çelik, L.Y. Koc, M.L. Yola, Z. Üstündağ, Phosphorus SulfurSilicon Relat. Elem. 188, 1723 (2013)
S. Pawar, A. Amate, D. Chakravarty, R.J. Butcher, A.A. Kumbhar, J. Chem. Sci. 133, 1 (2021)
H. Muğlu, H. Yakan, A.G.A. Misbah, M.S. Çavuş, T.K. Bakır, Res. Chem. Intermed. 47, 4985 (2021)
H. Yakan, T.K. Bakır, M.S. Çavuş, H. Muğlu, Res. Chem. Intermed. 46, 5417 (2020)
H. Muğlu, H. Yakan, T.K. Bakir, Turk. J. Chem. 44, 237 (2020)
G. Kiran, M. Sarangapani, T. Gouthami, A.R. Narsimha Reddy, Toxicol. Environ. Chem. 95, 367 (2013)
B. Sharma, Instrumental Methods of Chemical Analysis, Krishna Prakashan Media (Goel Publishing House, Meerut, 2000)
K.H.D. Reddy, S.-M. Lee, K. Seshaiah, R.K. Babu, J. Serb. Chem. Soc. 78, 229 (2013)
G. Subhashree, J. Haribabu, S. Saranya, P. Yuvaraj, D.A. Krishnan, R. Karvembu, D. Gayathri, J. Mol. Struct. 1145, 160 (2017)
N. Kurita, K. Kobayashi, Comput. Chem. 24, 351 (2000)
S. Niroomand, M. Khorasani-Motlagh, M. Noroozifar, S. Jahani, A. Moodi, J. Mol. Struct. 1130, 940 (2017)
S. Singhal, P. Khanna, L. Khanna, Heliyon 5, e02596 (2019)
M. Erol, I. Celik, G. Kuyucuklu, J. Mol. Struct. 1234, 130151 (2021)
M. Salihović, M. Pazalja, S.Š Halilović, E. Veljović, I. Mahmutović-Dizdarević, S. Roca, I. Novaković, S. Trifunović, J. Mol. Struct. 1241, 130670 (2021)
N. Chouchène, A. Toumi, S. Boudriga, H. Edziri, M. Sobeh, M.A. Abdelfattah, M. Askri, M. Knorr, C. Strohmann, L. Brieger, Molecules 27, 582 (2022)
S. Farooq, Z. Ngaini, A.I. Daud, W.M. Khairul, Polycycl. Aromat. Compd. 42, 5422 (2022)
H.M. Metwally, N.A. Khalaf, E. Abdel-Latif, M.A. Ismail, BMC Chem. 17, 1 (2023)
M.B. Muluk, P.S. Phatak, S.B. Pawar, S.T. Dhumal, N.N. Rehman, P.P. Dixit, P.B. Choudhari, K.P. Haval, J. Chin. Chem. Soc. 66, 1507 (2019)
N. Asmafiliz, Z. Kılıc, A. Öztürk, T. Hökelek, L.Y. Koc, L. Açık, O.Z.L. Kısa, A. Albay, Z. Üstündağ, A.O. Solak, Inorg. Chem. 48, 10102 (2009)
G.D. Çelik, A. Dişli, Y. Öner, L. Açık, Chem. Pharm. Bull. 60, 578 (2012)
Acknowledgements
The DFT calculations reported in this paper were partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources). We would like to thank Dr. Seyhan Ozturk at Ondokuz Mayıs University for taking the UV-Vis spectra.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MSÇ: Theoretical Calculations, Writing–Review. HY: Spectroscopic Characterization, Writing–Review, Visualization & Editing. CB: Biological studies, Writing–Review. ME: Spectroscopic Characterization, Writing–Review. HM: Synthesis, Characterization, Writing–Review. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest a financial or personal nature.
Ethical approval
All of the material is owned by the authors, and/or no permissions are required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çavuş, M., Yakan, H., Başkan, C. et al. Thio/carbohydrazone derivatives from iso(thio)/cyanates: preparation, structure elucidation, DFT studies, antimicrobial activity and DNA interactions. Res Chem Intermed 49, 2639–2667 (2023). https://doi.org/10.1007/s11164-023-05014-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05014-6