Abstract
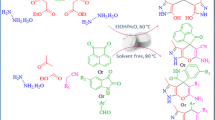
A facile, eco-friendly, and efficient approach for the multicomponent synthesis of 2-amino-pyran analogues (4a–j) is described that involves the reaction of substituted aldehydes, methyl cyanoacetate, and 1,3-cyclohexadione in a one-pot method using ruthenia-doped alumina (RuO2/Al2O3) as heterogeneous catalyst in a green solvent system. A simple wet-impregnation approach was used to prepare the catalyst material and was well-characterized using several analytical techniques like PXRD, TEM, SEM, SEM–EDX, and BET analysis. The key benefits of the current protocol are operational simplicity, economy, green reaction conditions, easy workup, short reaction time (10 min), higher product yields (94–98%), and no need for column chromatographic purification. The additional key advantage of this method is the recyclability and reusability of catalyst material up to eight runs through simple filtration without any significant loss of its catalytic activity.








Similar content being viewed by others
References
Ch. Madhavi, H. Ganja, N. Kerru, S. Maddila, S.B. Jonnalagadda, Appl. Organomet. Chem. 34, e6442 (2021). https://doi.org/10.1002/aoc.6442
N. Kerru, S. Maddila, S.B. Jonnalagadda, Front. Chem. 9, 638832 (2021). https://doi.org/10.3389/fchem.2021.638832
K.K. Gangu, J.V.S.K.V. Kalyani, T.S. Guru, S. Maddila, S.B. Jonnalagadda, Mater. Today Commun. 28, 102206 (2021)
N. Kerru, S. Maddila, S.B. Jonnalagadda, Curr. Org. Chem. 23, 3156 (2019). https://doi.org/10.2174/1385272823666191202105820
N. Kerru, L. Gummidi, S. Maddila, S.B. Jonnalagadda, Curr. Org. Chem. 25, 1 (2021). https://doi.org/10.2174/1385272824999201020204620
D.J. Rao, N. Kerru, S. Maddila, Chem. Data Collect. 32, 100669 (2021). https://doi.org/10.1016/j.cdc.2021.100704
N. Kerru, L. Gummidi, S. Maddila, S.B. Jonnalagadda, Inorg. Chem. Commun. 123, 108321 (2021). https://doi.org/10.1016/j.inoche.2020.108321
S. Harikrishna, A.R. Robert, H. Ganja, S. Maddila, S.B. Jonnalagadda, Sustain. Chem. Pharm. 16, 100265 (2020). https://doi.org/10.1016/j.scp.2020.100265
M. Costa, T.A. Dias, A. Brito, F. Proenca, Eur. J. Med. Chem. 123, 487 (2016). https://doi.org/10.1016/j.ejmech.2016.07.057
S. Maddila, S. Gorle, S.B. Jonnalagadda, Expert Opin. Drug Discov. 15, 203 (2020). https://doi.org/10.1080/17460441.2020.1696768
N. Kerru, V.H.S.S. Bhaskaruni, R. Kishore, S. Maddila, S.B. Jonnalagadda, Lett. Drug Des. Discov. 15, 118 (2018). https://doi.org/10.2174/1570180814666170710161844
Y.D. Duan, Y.Y. Jiang, F.X. Guo, L.X. Chen, L.L. Xu, W. Zhang, B. Liu, Fitoterapia 135, 114 (2019). https://doi.org/10.1016/j.fitote.2019.04.012
F.M. Wunsch, B. Wünsch, F.A. Bernal, T.J. Schmidt, Molecules 26, 5249 (2021). https://doi.org/10.3390/molecules26175249
C. Hu, L. Jiang, L. Tang, M. Zhang, R. Sheng, Bioorg. Med. Chem. (2021). https://doi.org/10.1021/jm800869t
N.A. Farag, S.R. Mohamed, G.A. Soliman, Bioorg. Med. Chem. 16, 9009 (2008). https://doi.org/10.1016/j.bmc.2008.08.039
P. Lerdsirisuk, C. Maicheen, J. Ungwitayatorn, Bioorg. Chem. 57, 142 (2014). https://doi.org/10.1016/j.bioorg.2014.10.006
B. Gopishetty, S. Hazeldine, S. Santra, M. Johnson, G. Modi, S. Ali, J. Zhen, M. Reith, A. Dutta, F J. Med. Chem. 54, 2924 (2011). https://doi.org/10.1021/jm200020a
L.Y. Zeng, B. Xi, K. Huang, J. Bi, L. Wei, C. Cai, S. Liu, ACS Comb Sci. 21, 656 (2019). https://doi.org/10.1021/acscombsci.9b00050
A.R. Saundane, K. Vijaykumar, A.V. Vaijinath, Bioorg. Med. Chem. Lett. 23, 1978 (2013). https://doi.org/10.1016/j.bmcl.2013.02.036
K. Nicole, C. Gianluca, M. Domenico, L. Erik, P. Sabrina, V.C. Carlo, H. Hans-Ulrich, A. Carmen, R.N. Francisco, S. Dirk, W. Bernhard, Eur. J. Med. Chem. 219, 113443 (2021). https://doi.org/10.1016/j.ejmech.2021.113443
B. Saeed, B. Morteza, S.A. Masoumeh, Syn. Commun. 37, 1097 (2007)
S.F. Hojati, N.M. Eghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018). https://doi.org/10.1080/00304948.2018.1468982
K. Khandan-Barani, M. Kangani, M. Mirbaluchzehi, Z. Siroos, Inorg. Nano-Met. Chem. 47, 751 (2017). https://doi.org/10.1080/15533174.2016.1212233
M. Aghaei-Hashjin, A. Yahyazadeh, E. Abbaspour-Gilandeh, RSC Adv. 11, 23491 (2021). https://doi.org/10.1039/D1RA04381A
S. Saneinezhad, L. Mohammadi, V. Zadsirjan, F.F. Bamoharram, M.M. Heravi, Sci. Rep. 10, 1 (2020). https://doi.org/10.1038/s41598-020-70738-z
R. Rahnamafa, L. Moradi, M. Khoobi, Res. Chem. Intermed. 46, 2109 (2020). https://doi.org/10.1007/s11164-020-04081-3
P.B. Hiremath, K. Kantharaju, ChemistrySelect 5, 1896 (2020). https://doi.org/10.1002/slct.201904336
D. Tahmassebi, J.E. Blevins, J.S. Gerardot. Appl. Organomet. Chem. 33, e4807 (2019). https://doi.org/10.1002/aoc.4807
A. Jamshidi, B. Maleki, F.M. Zonoz, R. Tayebee, Mater. Chem. Phys. 209, 46 (2018). https://doi.org/10.1016/j.matchemphys.2018.01.070
S.V.H.S. Bhaskaruni, K.K. Gangu, S. Maddila, S.B. Jonnalagadda, Chem. Rec. 19, 1793 (2019). https://doi.org/10.1002/tcr.201800077
H. Ganja, A.R. Robert, P. Lavanya, S. Chinnam, S. Maddila, S.B. Jonnalagadda, Inorg. Chem. Commun. 114, 107 (2020). https://doi.org/10.1016/j.inoche.2020.107807
S.V.H.S. Bhaskaruni, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Arab. J. Chem. 13, 1142 (2020). https://doi.org/10.1016/j.arabjc.2017.09.016
S. Harikrishna, A.R. Robert, H. Ganja, S. Maddila, S.B. Jonnalagadda. Appl. Organomet. Chem. 34, e5796 (2020). https://doi.org/10.1002/aoc.5796
S.N. Maddila, S. Maddila, N. Kerru, S.V.H.S. Bhaskaruni, S.B. Jonnalagadda, ChemistrySelect 5, 1786 (2020). https://doi.org/10.1002/slct.201901867
S.V.H.S. Bhaskaruni, S. Maddila, W.E. Van Zyl, S.B. Jonnalagadda, Catal. Commun. 100, 24 (2017). https://doi.org/10.1016/j.catcom.2017.06.023
S. Shabalala, S. Maddila, W.E. Van Zyl, S.B. Jonnalagadda, ACS-Ind. Eng. Chem. Res. 56, 11372 (2017). https://doi.org/10.1016/j.psep.2022.01.054
K.K. Gangu, S. Maddila, S.N. Maddila, S.B. Jonnalagadda, RSC Adv. 7, 423 (2017). https://doi.org/10.1039/C6RA25372E
X. Pan, F. Jiao, D. Miao, X. Bao, Chem Rev. 121, 6588 (2021). https://doi.org/10.1021/acs.chemrev.0c01012
Q. Song, W.D. Wang, X. Hu, Z. Dong, Nanoscale 11, 21513 (2019). https://doi.org/10.1039/C9NR08483E
W. Wang, M. Xu, X. Xu, W. Zhou, Z. Shao, Angew Chem. Int. Ed. Engl. 59, 136 (2020). https://doi.org/10.1002/anie.201900292
C. Theunissen, M.A. Ashley, T. Rovis, J. Am. Chem. Soc. 141, 6791 (2019). https://doi.org/10.1021/jacs.8b13663
S. Chen, A.M. Abdel-Mageed, D. Li, J. Bansmann, S. Cisneros, J. Biskupek, W. Huang, R.J. Behm, Angew Chem. Int. Ed. Engl. 58, 10732 (2019). https://doi.org/10.1002/anie.201903882
R.L. Arevalo, S.M. Aspera, M.C. SisonEscano, H. Nakanishi, H. Kasai, ACS Omega 2, 1295 (2017). https://doi.org/10.1021/acsomega.6b00462
S. Maddila, S. Gorle, S. Shabalala, O. Oyetade, S.N. Maddila, P. Lavanya, S.B. Jonnalagadda, Arab. J. Chem. 12, 671 (2019). https://doi.org/10.1016/j.arabjc.2016.04.016
M.R. Khumalo, S.N. Maddila, S. Maddila, S.B. Jonnalagadda, ChemistrySelect 4, 12503 (2019). https://doi.org/10.1002/slct.201903222
M.R. Khumalo, S.N. Maddila, S. Maddila, S.B. Jonnalagadda, RSC Adv. 9, 30768 (2019). https://doi.org/10.1039/C9RA04604F
N. Kerru, L. Gummidi, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Molecules 25, 1909 (2020). https://doi.org/10.3390/molecules25081909
S. Gorle, K.K. Gangu, S. Maddila, S.B. Jonnalagadda, Chem. Data Collect. 28, 100 (2020). https://doi.org/10.1016/j.cdc.2020.100471
D. Zhenhua, L. Xiaohua, F. Juhua, W. Min, L. Lili, F. Xiaoming, Eur. J. Org. Chem. 1, 137 (2011). https://doi.org/10.1002/ejoc.201001151
B. Saeed, B. Morteza, M. Sheikh-Ahmadi, S. Hekmat, P. Salehi, Syn. Commun. 37(7), 1097 (2007). https://doi.org/10.1080/00397910701196579
L. Rong, X. Li, H. Wang, D. Shi, S. Tu, Q. Zhuang, Synth. Commun. 36, 2363 (2006). https://doi.org/10.1080/003979106006402302363
W. Xiang-Shan, S. Da-Qing, T. Shu-Jiang, Y. Chang-Sheng, Synth. Commun. 33(1), 119 (2003). https://doi.org/10.1081/SCC-120015567
L. Ji-Tai, X. Wen-Zhi, Y. Li-Chao, L. Tong-Shuang, Synth. Commun. 34(24), 4565 (2004). https://doi.org/10.1081/SCC-200043233
R. Naresh, A. Santhi, D. Derong, A. Hadi, Z. John, C.-G.J. Heterocyc, Chemistry 54(1), 677 (2017). https://doi.org/10.1002/jhet.2641
H. Alireza, S. Mohsen, G. Nooshin, Z. Abdolkarim, D.M. Mohammad, Appl. Catal. A Gen. 402(1–2), 11 (2011). https://doi.org/10.1016/j.apcata.2011.04.012
Acknowledgements
The authors are very thankful to the Andhra University, and GITAM Deemed to be University, Visakhapatnam, India, for instrumentation, research, and financial support.
Funding
This work received no fund from any source.
Author information
Authors and Affiliations
Contributions
BA performed experimental studies and conceptualization; ARR performed writing—original draft and conceptualization; MMKK provided facilities for the spectral characterization; RM provided facilities for the catalyst characterization; PM performed validation, data curation, and formal analysis; SM: performed conceptualization, project administration, writing—original draft, supervision and funding acquisition; SBJ performed review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Apparao, B., Robert, A.R., Kumar, M.M.K. et al. Design of novel 2-amino-pyrans via a green and facile one-pot multicomponent protocol using RuO2/Al2O3 as reusable catalyst. Res Chem Intermed 49, 1043–1058 (2023). https://doi.org/10.1007/s11164-022-04949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04949-6