Abstract
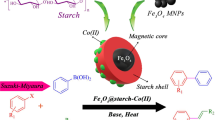
Bread scraps with transition metal oxide spinels were utilized to synthesize core–shell magnetic nanocatalyst. The prepared heterogeneous catalyst was characterized using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), thermogravimetric analysis (TGA), and transmission electron microscopy (TEM). This magnetic nanocatalyst opens up a new avenue to introduce a very useful and efficient catalytic system for the one-pot synthesis of tetraketone derivatives via Knoevenagel-Micheal reaction of 1,3 cyclohexanedione and benzaldehyde in ethanol as a green solvent at 50 °C, and one-pot four-component reaction of benzaldehyde, dimedone, ethyl acetoacetate, and hydrazine hydrate via tandem Knoevenagel-Micheal reaction for the synthesis of dihydropyrano[2,3-c] pyrazole derivatives in ethanol. The pharmaceutical properties of dihydropyrano[2,3-c] pyrazole and tetraketones make them both essential compounds. Moreover, the recyclability of the new heterogeneous magnetic catalyst does not significantly deteriorate its catalytic activity, and it can be easily recovered by an external magnet and reused ten times without significant loss of activity, making it environmentally friendly and economically feasible to perform the desired transformations. NBO calculations were carried out at the level of B3LYP/6–311 + + G (d, p) to obtain the atoms' natural charge data to justify the reaction's mechanism.



















Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
References
L. Chen, Y. Dai, H. Hou, W. Wang, X. Ding, H. Zhang, X. Li, H. Dong, Food Hydrocoll. 115, 106606 (2021)
R.N. Baig, R.S. Varma, Chem. comm. 49, 752 (2013)
A.H. Cahyana, A.R. Liandi, M. Maghdalena, R.T. Yunarti, T.P. Wendari, Ceram. Int. 48, 18316 (2022)
A.H. Cahyana, A.R. Liandi, Y. Yulizar, Y. Romdoni, T.P. Wendari, Ceram. Int. 47, 21373 (2021)
A.R. Liandi, A.H. Cahyana, R.T. Yunarti, T.P. Wendari, Ceram. Int. 48, 20266 (2022)
S.B. Kalidindi, B.R. Jagirdar, Chem. Sus. Chem. 5, 65 (2012)
R. Narayanan, Green Chem. Lett. Rev 5, 707 (2012)
Y. Xu, H. Zhang, X. Duan, Y. Ding, Mater. Chem. Phys. 114, 795 (2009)
Z. Zi, Y. Sun, X. Zhu, Z. Yang, J. Dai, W. Song, J. Magn. Magn. Mater. 321, 1251 (2009)
D.S. Mathew, R.-S. Juang, J. Chem. Eng. 129, 51 (2007)
Q. Yang, H. Choi, S.R. Al-Abed, D.D. Dionysiou, Appl. Catal. B. 88, 462 (2009)
R. Redon, N.G.G. Pena, F.R. Crescencio, Recent Pat. Nanotechnol. 8, 31 (2014)
W. Zhu, Z. Chen, Y. Pan, R. Dai, Y. Wu, Z. Zhuang, D. Wang, Q. Peng, C. Chen, Y. Li, Adv. Mater. 31, 1800426 (2019)
J.M. Khurana, K. Vij, J. Chem. Sci. 124, 907 (2012)
Z. Ren, W. Cao, W. Tong, X. Jing, Synth. Commun. 32, 1947 (2002)
F. Karimi Rad, F.K. Behbahani, Curr. Org. Synth. 14, 22 (2017)
P. Zhang, Y.-D. Yu, Z.-H. Zhang, Synth. Commun. 38, 4474 (2008)
A.S. Patki, K.N. Patil, S. Kusuma, D.B. Muley, A.H. Jadhav, Res. Chem. Intermed. 47, 2751 (2021)
S.R. Mathapati, K.N. Patil, S.S. Mathakari, A.W. Suryawanshi, A.H. Jadhav, Phosphorus Sulfur Silicon Relat. Elem. 196, 538 (2021)
S.R. Mathapati, R.C. Alange, C. Sherin Mol, S.S. Bhande, A.H. Jadhav, Res. Chem. Intermed. 48, 4901 (2022)
M.B. Swami, A.H. Jadhav, S.R. Mathpati, H.G. Ghuge, S.G. Patil, Res. Chem. Intermed. 43, 2033 (2017)
F. Mohamadpour, J. Chem. Sci. 132, 1 (2020)
A.R. Moosavi-Zare, M.A. Zolfigol, A. Mousavi-Tashar, Res. Chem. Intermed. 42, 7305 (2016)
S.R. Attar, B. Shinde, S.B. Kamble, Res. Chem. Intermed. 46, 4723 (2020)
B. Maleki, M. Raei, H. Alinezhad, R. Tayebee, A. Sedrpoushan, Org. Prep. Proced. Int. 50, 288 (2018)
D. Kumar, J.S. Sandhu, Synth. Commun. 40, 510 (2010)
M. Saha, J. Dey, K. Ismail, A.K. Pal, Lett. Org. Chem. 8, 554 (2011)
M. Fallah, H. Tajbakhsh, A. Vahedi, Bekhradnia. Res. Chem. Intermed. 43, 29 (2017)
D. Shi, Y. Wang, Z. Lu, G. Dai, Synth. Commun. 30, 707 (2000)
S. Yokote, S. Nishikawa, K. Shibuya, K. Hisano, H. Nishino, Tetrahedron 76, 131165 (2020)
H. Hassanzadeh-Afruzi, F. Dogari, A. Esmailzadeh, Maleki. Appl. Organomet. Chem. 35, e6363 (2021)
R.J. Cremlyn, A.G. Osborne, J.F. Warmsley, Spectrochim. Acta - A: Mol. Biomol. 52, 1423 (1996)
Y. Fu, B. Fan, H. Chen, H. Huang, Y. Hu, Bioorg. Chem. 80, 555 (2018)
J. Safaei-Ghomi, H. Shahbazi-Alavi, E. Heidari-Baghbahadorani, J. Chem. Res. 39, 410 (2015)
S.N. Maddila, S. Maddila, W.E. van Zyl, S.B. Jonnalagadda, Res. Chem. Intermed. 43, 4313 (2017)
M. Nikoorazm, B. Tahmasbi, S. Gholami, P. Moradi, Appl. Organomet. Chem. 34, e5919 (2020)
N. Salehi, B.B.F. Mirjalili, Org. Prep. Proced. Int. 50, 578 (2018)
F. Abdel-Latif, M. Mashaly, R. Mekheimer, T. Abdel-Aleem, Z. Naturforsch. B. 48, 817 (1993)
D. Thakur, H.S. Sohal, Eur. J. Mol. Clin. Med. 7, 4498 (2020)
B. Ardiansah, Int. J. Chemtech Res. 12, 273 (2019)
M. Zabihzadeh, F. Shirini, H. Tajik, N. Daneshvar, Polycycl. Aromat. Compd. 41, 1972 (2021)
S. Srivastava, ChemistrySelect 5, 799 (2020)
S. Arora, G. Joshi, S. Kalra, A.A. Wani, P.V. Bharatam, P. Kumar, R. Kumar, ACS Omega 4, 4604 (2019)
A. Ziyaei Halimehjani, V. Barati, M. Karimi, Synth. Commun. 49, 724 (2019)
B.M. Sapkal, P.K. Labhane, J.R. Satam, Res. Chem. Intermed. 43, 4967 (2017)
Funding
We gratefully acknowledge the funding support received for this project from the Sharif University of Technology (SUT), Islamic Republic of Iran.
Author information
Authors and Affiliations
Contributions
FMM contributed to project administration, supervision, conceptualization, validation, resources, and funding acquisition. SA contributed to methodology, formal analysis, funding acquisition, visualization, data curation, software, and writing—original draft. MD contributed to data curation, formal analysis, investigation, methodology, writing—original draft, validation, and conceptualization. HM contributed to methodology, formal analysis, and validation. ZD contributed to data curation, formal analysis, conceptualization, investigation, methodology, writing—original draft, visualization, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moghaddam, F.M., Aghili, S., Daneshfar, M. et al. Bread waste in the form of CoFe2O4@TBW catalyst was used as a green biocatalyst to synthesize pyranopyrazole and tetraketone derivatives. Res Chem Intermed 49, 1507–1543 (2023). https://doi.org/10.1007/s11164-022-04934-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04934-z