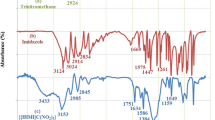
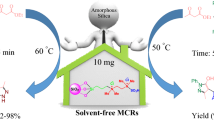
Abstract
In this study, glycine-based ionic liquid ([Gly-H][OTs]) was synthesized through a reaction of glycine with ρ-toluenesulfonic acid in water. The synthesized IL was utilized for the one-pot, multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones, 14-aryl-14H-dibenzo[a,j]xanthenes and 12-aryl-8,9,10,12-tetrahydrobenzo [a]xanthene-11-ones under solvent-free condition. The prepared IL indicates desirable catalytic activity in organic reactions under solvent-free conditions with the high reusability of four runs. The proposed protocol's main advantages are the originality of prepared [Gly-H][OTs], organic green synthesis under solvent-free conditions, high product yields, and less reaction times.











Similar content being viewed by others
Data availability
Not applicable.
References
T. Welton, Biophys. Rev. 10, 691 (2018)
K. Sood, Y. Saini, K.K. Thakur, Mater. Today Proc. (2021 in press)
M. Salami, A. Ezabadi, Polycycl. Aromat. Compd. 42, 3377 (2022)
D. Liu, H. Wang, M. Liang, Y. Nie, Y. Liu, M. Yin, X. Qiao, J. Chromatogr. A 1660, 462676 (2021)
J.-M. Leveque, J. Estager, M. Draye, G. Cravotto, L. Boffa, W. Bonrath, Monatsh. Fur Chem. 138, 1103 (2007)
D.A. Waterkamp, M. Heiland, M. Schlüter, J.C. Sauvageau, T. Beyersdorff, J. Thöming, Green Chem. 9, 1084 (2007)
Z. Radai, N.Z. Kiss, G. Keglevich, Curr. Org. Chem. 22, 533 (2018)
Z. Yang, C. Sun, C. Zhang, S. Zhao, M. Cai, Z. Liu, Q. Yu, Tribol. Int. 153, 106663 (2021)
Y. Qu, J. Lan, Y. Chen, J. Sun, Sustain. Energy Fuels 5, 2494 (2021)
R. Aslam, M. Mobin, M. Murmu, P. Banerjee, J. Aslam, J. Mol. Liq. 334, 116469 (2021)
N. Sharma, U.K. Sharma, R. Kumar, A.K. Sinha, RSC Adv. 2, 10648 (2012)
V.V. Chaban, E.E. Fileti, (2014)
A.S. Amarasekara, Chem. Rev. 116, 6133 (2016)
H. Olivier-Bourbigou, L. Magna, D. Morvan, Appl. Catal. A-Gen. 373, 1 (2010)
K. Lam, H. Timmerman, Drug Discov. Today Technol. 29, 103420 (2018)
S. Javanbakht, A. Shaabani, A.C.S. Appl, Bio Mater. 3, 156 (2019)
S. Zhi, X. Ma, W. Zhang, Org. Biomol. Chem. 17, 7632 (2019)
O. Soleimani, J. Chem. Rev. 2, 169 (2020)
L.H.S. Matos, F.T. Masson, L.A. Simeoni, M. Homem-de-Mello, Eur. J. Med. Chem. 143, 1779 (2018)
N.C. Desai, S.B. Joshi, K.A. Jadeja, J. Heterocycl. Chem. 57, 791 (2020)
A.N. Panaskar, A. Jain, P.K. Mohanty, J. Pharm. Res. Int. 33, 481 (2021)
K. Jeena-Thomas, M. George, L. Joseph, J. Chem. Res. 4, 19 (2019)
J. Dowarah, D. Patel, B.N. Marak, U.C.S. Yadav, P.K. Shah, P.K. Shukla, V.P. Singh, RSC Adv. 11, 35737 (2021)
E. Guantai, K. Chibale, Curr. Drug Deliv. 7, 312 (2010)
K.M. Bairagi, N.S. Younis, P.M. Emeka, E. Sangtani, R.G. Gonnade, K.N. Venugopala, O.I. Alwassil, H.E. Khalil, S.K. Nayak, Med. Chem. 16, 996 (2020)
B. Gawdzik, P. Kowalczyk, D. Koszelewski, A. Brodzka, J. Masternak, K. Kramkowski, A. Wypych, R. Ostaszewski, Membranes 12, 238 (2022)
C.O. Kappe, Q.S.A.R. Comb, Sci. 22, 630 (2003)
C.O. Kappe, Acc. Chem. Res. 33, 879 (2000)
C.O. Kappe, Tetrahedron 49, 6937 (1993)
K.M. Hua, P.H. Tran, T.N. Le, Arkivoc 6, 406 (2019)
N.D. Kadam, R.V. Jayaram, Curr. Catal. 7, 52 (2018)
M. Aalam, S. Singh, ChemistrySelect 7, 202103918 (2022)
B.-J. Yao, W.-X. Wu, L.-G. Ding, Y.-B. Dong, J. Org. Chem. 86, 3024 (2021)
M. Kaur, D. Bains, G. Singh, N. Kaur, N. Singh, Polym. Chem. 12, 1165 (2021)
S. Ghosh, N. Nagarjun, S. Nandi, A. Dhakshinamoorthy, S. Biswas, J. Mater. Chem. C 10, 6717 (2022)
M.R. Maurya, A. Chauhan, S. Arora, P. Gupta, Catal. Today 379–399, 3 (2022)
R. Singh, in Applications of nanotechnology for green synthesis, (Springer, 2020), p. 60
D. Fazzi, C. Castiglioni, F. Negri, Phys. Chem. Chem. Phys. 12, 1600 (2010)
X. Guo, X.-R. Wei, R. Sun, Y.-J. Xu, Y. Chen, J.-F. Ge, Sens. Actuators B Chem. 296, 126621 (2019)
G. Shabir, A. Saeed, P. Ali Channar, Mini Rev. Org. Chem. 15, 166 (2018)
T. Bezrodna, V. Bezrodnyi, A. Negriyko, L. Kosyanchuk, O. Antonenko, Macromol. Symp. 396, 2000232 (2021)
M. Pishgar, K. Gharanjig, M. Yazdanshenas, K. Farizadeh, A. Rashidi, Prog. Color. Color. Coat. 15, 87 (2022)
Z. Ehsani-Nasab, A. Ezabadi, Comb. Chem. High Throughput Screen. 21, 602 (2018)
H. Naeimi, Z. Ansarian, J. Taiwan Inst. Chem. Eng. 85, 265 (2018)
Z.A. Piralghar, M.M. Hashemi, A. Ezabadi, Polycycl. Aromat. Compd. 40, 1510 (2019)
H. Faroughi Niya, N. Hazeri, M. Fatahpour, M.T. Maghsoodlou, Res. Chem. Intermed. 46, 3651 (2020)
A. Marandi, N. Koukabi, M.A. Zolfigol, Res. Chem. Intermed. 47, 3145 (2021)
M. Zabihzadeh, F. Shirini, H. Tajik, N. Daneshvar, Polycycl. Aromat. Compd. 1, 2052 (2020)
S. Askari, M.M. Khodaei, M. Jafarzadeh, Res. Chem. Intermed. 47, 2881 (2021)
W. Abo El-Yazeed, O. Hayes, A.I. Ahmed, J. Sol–Gel Sci. Technol. 99, 140 (2021)
M. Salami, A. Ezabadi, Res. Chem. Intermed. 45, 3673 (2019)
H. Sahebi, E. Konoz, A. Ezabadi, New J. Chem. 43, 13554 (2019)
Z.A. Piralghar, M.M. Hashemi, A. Ezabadi, Comb. Chem. High Throughput Screen 21, 526 (2018)
R. Manepalli, G. Giridhar, P. Pardhasaradhi, P. Jayaprada, M. Tejaswi, K. Sivaram, C.M. Kumar, V. Pisipati, Mater. Today Proc. 5, 2666 (2018)
S. Saikia, R. Borah, ChemSelect 4, 8751 (2019)
L. Ouyang, T. Zheng, L. Shen, Cryst. Eng. Commun. 20, 2705 (2018)
Z. Karimi-Jaberi, M.S. Moaddeli, ISRN Org. Chem. 2012, 2154 (2012)
S. Gore, S. Baskaran, B. Koenig, Green Chem. 13, 1009 (2011)
M. Salami, A. Ezabadi, Res. Chem. Intermed. 46, 4611 (2020)
M. Salami, A. Ezabadi, Polycycl. Aromat. Compd. 1, 1245 (2020)
S.E. Sadati Sorkhi, M.M. Hashemi, A. Ezabadi, Res. Chem. Intermed. 46, 2229 (2020)
F. Shirini, M. Abedini, M. Seddighi, O.G. Jolodar, M. Safarpoor, N. Langroodi, S. Zamani, RSC Adv. 4, 63526 (2014)
F. Shirini, M.S.N. Langarudi, M. Seddighi, O.G. Jolodar, Res. Chem. Intermed. 41, 8483 (2015)
H. Valizadeh, A. Shockravi, Heteroat. Chem. Int. J. Main Group Elem. 20, 284 (2009)
B. Pouramiri, R. Fayazi, E. Tavakolinejad-Kermani, Iran. J. Chem. Chem. Eng. (IJCCE) 37, 159 (2018)
Z. Liu, R. Ma, D. Cao, C. Liu, Molecules 21, 462 (2016)
D. Fang, Z.L. Liu, J. Heterocycl. Chem. 47, 509 (2010)
K. Gong, D. Fang, H.-L. Wang, X.-L. Zhou, Z.-L. Liu, Dyes Pigm. 80, 30 (2009)
H. Naeimi, Z.S. Nazifi, C. R. Chim. 17, 41 (2014)
M. Zakeri, M.M. Heravi, M. Saeedi, N. Karimi, H.A. Oskooie, N. Tavakoli-Hoseini, Chin. J. Chem. 29, 1441 (2011)
X.-D. Jia, S.-Y. Han, H.-F. Duan, Y.-J. Lin, J.-G. Cao, D.-P. Liang, M.-C. Wu, Chem. Res. Chin. Univ. 29, 82 (2013)
H. Singh, S. Kumari, J.M. Khurana, Chin. Chem. Lett. 25, 1336 (2014)
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AE contributed to conceptualization, design the experiment, supervision, formal analysis, validation, and critical revision of the article. EH contributed to performing the experiment, data acquisition, formal analysis, initial draft, and revision of the article. ASA contributed to consulting on performing the experiment and revision of the article.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hataminejad, E., Ezabadi, A. & Shameli Akandi, A. Novel synthesis of nano-amino acid-based ionic liquid and its application for preparing DHPMs and xanthenes under solvent-free conditions. Res Chem Intermed 49, 1275–1295 (2023). https://doi.org/10.1007/s11164-022-04931-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04931-2