Abstract
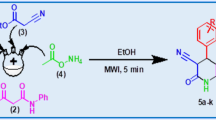
2-Amino-4H-benzo[H]chromene-3-carbonitrile and 2-amino-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile are two important pharmacophores with extensive applications in medicinal chemistry. The multicomponent Knoevenagel–Michael reaction is one of the most efficient strategies to construct these two scaffolds. Previous efforts have been made in converting the traditional organic solvents to eco-friendly solvents including H2O generally in the presence of complex catalysts. Here we present our work of using sodium fluoride (NaF) as the catalyst to synthesize a wide scope of the two types of substrates starting from α- or β-naphthol or 4-hydroxycoumarin. Using 12 mol% NaF under the microwave irradiation condition, the reactions were completed within 15–25 min with all the yields exceeding 85%. The reaction products were purified simply by washing with H2O and can be further purified by crystallization with MeOH. The analysis of green chemistry-related parameters suggested that the current method was highly environmentally benign, highlighting the potential of this method in the use of drug discovery and development.
Graphical abstract







Similar content being viewed by others
Data availability
The online version contains supplementary material.
References
G. Feuer, in Progress in Medicinal Chemistry, eds. G.P. Ellis, G.B West, 1st edn, vol. 10 (North-Holland Publishing Company, New York, 1974), p. 85
F.M. Dean, Naturally Occurring Oxygen Ring Compounds, vol. 200 (Butterworth-Heinemann, London, 1963), p.176
A. Goel, V.J. Ram, Tetrahedron 65, 7865 (2009)
F. Schmitt, M. Gold, M. Rothemund, I. Andronache, B. Biersack, R. Schobert, T. Mueller, Eur. J. Med. Chem. 163, 160 (2019)
G. Zhang, Y. Zhang, J. Yan, R. Chen, S. Wang, Y. Ma, R. Wang, J. Org. Chem. 77, 878 (2012)
A.P. Sarkate, V.S. Dofe, S.V. Tiwari, D.K. Lokwani, K.S. Karnik, D.D. Kamble, M.H.S.H. Ansari, S. Dodamani, S.S. Jalalpure, J.N. Sangshetti, R. Azad, P.V.L.S. Burra, S.V. Bhandari, Bioorg. Med. Chem. Lett. 40, 127916 (2021)
M. Khoobi, M. Alipour, A. Sakhteman, H. Nadri, A. Moradi, M. Ghandi, S. Emami, A. Foroumadi, A. Shafiee, Eur. J. Med. Chem. 68, 260 (2013)
A.S. Abd-El-Aziz, A. Alsaggaf, E. Assirey, A. Naqvi, R.M. Okasha, T.H. Afifi, M. Hagar, Int. J. mol. Sci. 22, 2807 (2021)
A.M. El Agrody, A.M. Fouda, E.S.A.E.H. Khattab, Med. Chem. Res. 22, 6105 (2013)
A.M. El Agrody, A.M. Fouda, E.S.A.E.H. Khattab, Med. Chem. Res. 26, 691 (2017)
M.R. Bhosle, D.B. Wahul, G.M. Bondle, A. Sarkate, S.V. Tiwari, Synth. Commun. 48, 2046 (2018)
B.M. Chougala, S. Samundeeswari, M. Holiyachi, N.S. Naik, L.A. Shastri, S. Dodamani, S. Jalalpure, S.R. Dixit, S.D. Joshi, V.A. Sunagar, Eur. J. Med. Chem. 143, 1744 (2018)
H.E.A. Ahmed, M.A.A. El-Nassag, A.H. Hassan, H.M. Mohamed, A.H. Halawa, R.M. Okasha, S. Ihmaid, S.M.A. El-Gilil, E.S.A.E.H. Khattab, A.M. Fouda, A.M. El-Agrody, A. Aljuhani, T.H. Afifi, J Mol Struct 1186, 212 (2019)
M. Mashhadinezhad, M. Mamaghani, M. Rassa, F. Shirini, ChemistrySelect 4, 4920 (2019)
R. Kaur, F. Naaz, S. Sharma, S. Mehndiratta, M.K. Gupta, P.M.S. Bedi, K. Nepali, Med. Chem. Res. 24, 3334 (2015)
M.M. Khafagy, A.H.F. Abd El Wahab, F.A. Eid, A.M. El Agrody, Farmaco 57, 715 (2002)
F.F. Alblewi, R.M. Okasha, A.A. Eskandrani, T.H. Afifi, H.M. Mohamed, A.H. Halawa, A.M. Fouda, A.-A.M. Al-Dies, A. Mora, A.M. El-Agrody, Molecules 24, 1060 (2019)
A.M. El-Agrody, A.M. Fouda, A.-A.M. Al-Dies, Med. Chem. Res. 23, 3187 (2014)
F.F. Alblewi, R.M. Okasha, Z.M. Hritani, H.M. Mohamed, M.A.A. El-Nassag, A.H. Halawa, A. Mora, A.M. Fouda, M.A. Assiri, A.A.M. Al-Dies, T.H. Afifi, A.M. El-Agrody, Bioorg. Chem. 87, 560 (2019)
E. Maalej, F. Chabchoub, M.J. Oset-Gasque, M.E. Pérez, M.P. Gonzalez, L. Monjas, C. Pérez, C. Rios, M.I. Rodriguez-Franco, I. Iriepa, I. Moraleda, M. Chioua, A. Romero, J. Marco-Contelles, A. Samadi, Eur. J. Med. Chem. 54, 750 (2012)
H.R. Safaei, M. Shekouhy, S. Rahmanpur, A. Shirinfeshan, Green Chem. 14, 1696 (2012)
N.V. Shitole, K.F. Shelke, S.A. Sadaphal, B.B. Shingate, M.S. Shingare, Green Chem. Lett. Rev. 3, 83 (2010)
P. Li, Y. Yang, L. Mi, K. Gao, M. Tao, W. Chen, Catal. Lett. 151, 2056 (2021)
A.S. Patel, S.D. Tala, P.B. Nariya, K.D. Ladva, N.P. Kapuriya, J. Chin. Chem. Soc. 66, 247 (2019)
P. Mohammadi, H. Sheibani, Mater. Chem. Phys. 228, 140 (2019)
M. Khaleghi-Abbasabadi, D. Azarifar, Res. Chem. Intermed. 45, 2095 (2019)
M.S. Nejad, H. Sheibani, Catal. Lett. 148, 125 (2018)
B.K. Mayank, P.K. Billing, N. Agnihotri, N. Kaur, D.O. Singh, Jang, ACS Sustain. Chem. Eng. 6, 3714 (2018)
M.R. Yousefi, O. Goli-Jolodar, F. Shirini, Bioorg. Chem. 81, 326 (2018)
N. Daneshvar, O. Goli-Jolodar, R. Karimi-Chayjani, M.S.N. Langarudi, F. Shirini, ChemistrySelect 4, 1562 (2019)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 7847 (2015)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 4031 (2015)
P. Das, A. Dutta, A. Bhaumik, C. Mukhopadhyay, Green Chem. 16, 1426 (2014)
Y. Wang, C. Yue, X. Li, J. Luo, C.R. Chim. 19, 1021 (2016)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
S.M. Baghbanian, N. Rezaei, H. Tashakkorian, Green Chem. 15, 3446 (2013)
F.K. Behbahani, S. Maryam, J. Korean Chem. Soc. 57, 357 (2013)
E. Sheikhhosseini, D. Ghazanfari, V. Nezamabadi, Iran. J. Catal. 3, 197 (2013)
Y. Wang, J. Luo, T. Xing, Z. Liu, Monatsh. Chem. 144, 1871 (2013)
M. Khoobi, L. Ma’mani, F. Rezazadeh, Z. Zareie, A. Foroumad, A. Ramazani, A. Shafiee, J. Mol. Catal. A. Chem. 359, 74 (2012)
S. Samantaray, D.K. Pradhan, G. Hota, B.G. Mishra, Chem. Eng. J. 193, 1 (2012)
M.M. Heravi, M. Zakeri, N. Mohammadi, Chin. J. Chem. 29, 1163 (2011)
H. Mehrabi, N. Kamali, J. Iran. Chem. Soc. 9, 599 (2012)
H. Mehrabi, M. Kazemi-Mireki, Chin. Chem. Lett. 22, 1419 (2011)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7, 890 (2010)
J.M. Khurana, B. Nand, P. Saluja, Tetrahedron 66, 5637 (2010)
K. Gong, H.L. Wang, D. Fang, Z.-L. Liu, Catal. Commun. 9, 650 (2008)
P. Li, Y. Yang, X. Wu, J. Lu, L. Hu, W. Chen, W. Zhang, Catal. Lett. 152, 43 (2022)
M.M. Heravi, K. Bakhtiari, V. Zadsirjan, F.F. Bamoharram, O.M. Heravi, Bioorg. Med. Chem. Lett. 17, 4262 (2007)
A. Patra, T. Mahapatra, J. Chem. Res. 2008, 405 (2008)
L.Z. Fekri, M. Barazandehdoust, Polycycl. Aromat. Compds. 41, 1274 (2021)
S.T. Fardood, A. Ramazani, P.A. Asiabi, Y.B. Fard, B. Ebadzadeha, Asian J Green Chem. 1, 34 (2017)
F. Rahmatpour, M. Kosari, N. Monadi, J. Mol. Struct. 1253, 132102 (2022)
F. Kamali, F. Shirini, Eurasian Chem. Commun. 3, 278 (2021)
A. Pourkazemi, Z. Nasouri, F. Fakhraie, A. Razzaghi, A. Parhami, A. Zare, Asian J. Nanosci. Mater. 3, 131 (2020)
N. Divsalar, N. Monadi, M. Tajbaksh, J. Nanostruct. 6, 312 (2016)
M. Tajbakhsh, M. Kariminasab, H. Alinezhad, R. Hosseinzadeh, P. Rezaee, M. Tajbakhsh, H.J. Gazvini, M.A. Amiri, J. Iran. Chem. Soc. 12, 1405 (2015)
K.R. Desale, K.P. Nandre, S.L. Patil, Org. Commun. 5, 179 (2012)
B. Maleki, S. Sheikh, Org. Prep. Proced. Int. 47, 368 (2015)
M.A. Shaikh, M. Farooqui, S. Abed, Res. Chem. Intermed. 45, 1595 (2019)
M. Rohaniyan, A. Davoodnia, S.A. Beyramabadi, A. Khojastehnezhad, Appl. Organometal. Chem. 33, e4881 (2019)
H.R. Shaterian, M. Mohammadnia, Res. Chem. Intermed. 41, 1301 (2015)
J. Banothu, K. Vaarla, R. Bavantula, P.A. Crooks, Chem. Lett. 25, 172 (2014)
R. Konakanchi, R. Gondru, V.B. Nishtala, L.R. Kotha, Synth. Commun. 48, 1994 (2018)
R.C. Cioc, E. Ruijter, R.V.A. Orru, Green Chem. 16, 2958 (2014)
R.M.N. Kalla, I. Kim, Mol. Catal. 473, 110396 (2019)
Acknowledgements
FK and ACG thank the Ministry of Education (formerly known as the Ministry of Human Resource Development) for providing the fellowships. MC acknowledges the DST-INSPIRE (IF190417) for the fellowship. JB sincerely thanks the DST-SERB (EEQ/2020/000303) research grant and the Director, National Institute of Technology Calicut for the Faculty Research Seed Grant (FRG). All the authors are grateful to Dr. Yupeng Li, Research Assistant Professor, Department of Medicinal Chemistry, University of Minnesota, USA for his valuable suggestions. All the authors thankful to CMC, NIT Calicut for the characterization data and the Sophisticated Test and Instrumentation Centre, Cochin University of Science and Technology (STIC INDIA) for single crystal analysis.
Author information
Authors and Affiliations
Contributions
FK has carried out the experimental work and characterized all the compounds using spectroscopic studies. ACG collected all the references and wrote the draft of the manuscript. MC prepared and analyzed the single crystal structures of the two compounds. JB designed, executed and monitored the progress of the work, and corrected the draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fabitha, K., Arya, C.G., Chandrakanth, M. et al. Green chemistry approach: sodium fluoride-catalyzed highly efficient microwave irradiation-assisted synthesis of substituted chromene derivatives in aqueous medium. Res Chem Intermed 49, 997–1014 (2023). https://doi.org/10.1007/s11164-022-04929-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04929-w