Abstract
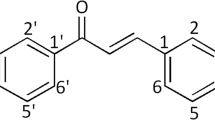
On the basis of the urgent need to develop innovative and safe anticancer compounds, a series of chalcones, functionalized with various substituents in the aromatic portions and a long N-alkyl amide moiety, were designed and synthesized. The compatibility of novel compounds was firstly evaluated on non-tumoral human gingival fibroblasts (HGFs), secondly, the anti-proliferative effect on an adenocarcinoma gastric cell line (AGS) was measured. Among the chalcones, compound 3, at 50 µM after 48 and 72 h, showed, at the same time, an appreciable capability to counteract tumoral AGS viability and to preserve a high rate of healthy HGFs viability. Cell cycle analysis, performed on AGS cell line with 3 subtoxic dose (30 µM), suggested that the anti-proliferative effect could be related to a cell cycle arrest during the first stage and to an increase in the oxidative stress demonstrated by the significant augmentation of Reactive Oxygen Species release. Collectively, we have demonstrated that chalcone 3 had a potent anti-proliferative potential for the treatment of gastric adenocarcinoma and could be further investigated for new drug development.








Similar content being viewed by others
Data availability
Not applicable.
References
S.C. Shah, V. Kayamba, R.M. Peek, D. Heimburger, J. Glob. Oncol. 5, 1 (2019)
H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray, CA Cancer J. Clin. 71, 209 (2021)
N.N. Pavlova, J. Zhu, C.B. Thompson, Cell Metab. 34, 355 (2022)
M. Růžička, P. Kulhánek, L. Radová, A. Čechová, N. Špačková, L. Fajkusová, K. Réblová, PLoS ONE 12, e0182377 (2017)
L. Falzone, S. Salomone, M. Libra, Front. Pharmacol. 9, 1300 (2018)
A.R. Kamal, J.S. Ramaiah, M.J. Dastagiri, D. Bharathi, E.V. Sagar, M.V. Pushpavalli, Med. Chem. Commun. 1, 355 (2010)
A. Musa, E.M. Mostafa, S.N.A. Bukhari, N.H. Alotaibi, A.H. El-Ghorab, A. Farouk, A.A. Nayl, M.M. Ghoneim, M.A. Abdelgawad, Molecules 27, 1158 (2022)
M. Sun, M. Yuan, Y. Kang, J. Qin, Y. Zhang, Y. Duan, L. Wang, Y. Yao, J. Enzyme Inhib. Med. Chem. 37, 339 (2022)
K. Sukumaran, R. Kuttan, J. Ethnopharmacol. 34, 93 (1991)
H.O. Tawfik, M.A. Shaldam, A. Nocentini, R. Salem, H. Almahli, S.T. Al-Rashood, C.T. Supuran, W.M. Eldehna, J. Enzyme Inhib. Med. Chem. 37, 1043 (2022)
L. Ni, C.Q. Meng, J.A. Sikorski, Expert Opin. Ther. Pat. 14, 1669 (2014)
C. Echeverria, J.F. Santibañez, O. Donoso-Tauda, C.A. Escobar, R. Ramirez-Tagle, Int. J. Mol. Sci. 10, 221 (2009)
A.-M. Katsori, D. Hadjipavlou-Litina, Curr. Med. Chem. 16, 1062 (2009)
T. Akihisa, H. Tokuda, M. Ukiya, M. Iizuka, S. Schneider, K. Ogasawara, T. Mukainaka, K. Iwatsuki, T. Suzuki, H. Nishino, Cancer Lett. 201, 133 (2003)
F. Moura, M.R.C. de Castro, R.F. Naves, A.J. Araújo, M.C.L. Dos Santos, J.D.B.M. Filho, C. Noda-Perez, F. Terra Martins, C.O. d0 Pessoa, M.O.M. Filho, Anticancer Agents Med. Chem. 22, 2340 (2021)
Y. Wang, L. Li, T. Ma, X. Cheng, D. Liu, Anticancer Agents Med. Chem. 22, 2116 (2021)
U. Shah, A. Patel, S. Patel, M. Patel, A. Patel, S. Patel, S. Patel, R. Maheshwari, A.G. Mtewa, K. Gandhi, Anticancer Agents Med. Chem. 22, 2063 (2021)
A. Joshi, H. Bhojwani, O. Wagal, K. Begwani, U. Joshi, S. Sathaye, D. Kanchan, Anticancer Agents Med. Chem. 22, 328 (2022)
S. Patil, S. Bhandari, Mini Rev. Med. Chem. 22, 805 (2021)
P.S. de Souza, G.C. Bibá, E.D. Melo, M.F. Muzitano, Nat. Prod. Res. (2021)
T. Constantinescu, C.N. Lungu, Int. J. Mol. Sci. 22, 11306 (2021)
V. Di Marzo, T. Bisogno, L. De Petrocellis, D. Melck, B.R. Martin, Curr. Med. Chem. 6, 721 (1999)
S. Burstein, R. Salmonsen, Bioorg. Med. Chem. 16, 9644 (2008)
S. Sarfaraz, V.M. Adhami, D.N. Syed, F. Afaq, H. Mukhtar, Cancer Res. 68, 339 (2008)
M.I. Chouiter, H. Boulebd, D.M. Pereira, P. Valentão, P.B. Andrade, A. Belfaitah, A.M. Silva, Future Med. Chem. 12, 493 (2020)
Y.C. Hseu, R.W. Lin, Y.C. Shen, K.Y. Lin, J.W. Liao, V. Thiyagarajan, H.L. Yang, Cancers 12, 2475 (2020)
F. Alibeiki, N. Jafari, M. Karimi, H. Peeri Dogaheh, Sci. Rep. 7, 2559 (2017)
X.Y. Xiao, M. Hao, X.Y. Yang, Q. Ba, M. Li, S.J. Ni, L.S. Wang, X. Du, Cancer Lett. 302, 69 (2011)
C.T. Chang, Y.C. Hseu, V. Thiyagarajan, K.Y. Lin, T.D. Way, M. Korivi, J.W. Liao, H.L. Yang, Arch. Toxicol. 91, 3341 (2017)
B.P. Bandgar, L.K. Adsul, H.V. Chavan, S.N. Shringare, B.L. Korbad, S.S. Jalde, Bioorg. Med. Chem 20, 5649 (2012)
N.M. Rateb, H.F. Zohdi, Synth. Commun. 39, 2789 (2009)
L.F. Pisani, G.E. Tontini, C. Gentile, B. Marinoni, I. Teani, N. Nandi, P. Creo, E. Asti, L. Bonavina, M. Vecchi, L. Pastorelli, Int. J. Mol. Sci. 22, 5792 (2021)
G. Sita, A. Graziosi, P. Hrelia, F. Morroni, Int. J. Mol. Sci. 22, 11201 (2021)
T. Liu, L. Sun, Y. Zhang, Y. Wang, J. Zheng, J. Biochem. Mol. Toxicol. 36, e22942 (2022)
M. De Colli, P. Tortorella, M. Agamennone, C. Campestre, F. Loiodice, A. Cataldi, S. Zara, Int. J. Mol. Med. 42, 651 (2018)
A. Ricci, M. Gallorini, D. Del Bufalo, A. Cataldi, I. D’Agostino, S. Carradori, S. Zara, Molecules 27, 957 (2022)
M.A. Alagöz, Z. Özdemir, M. Uysal, S. Carradori, M. Gallorini, A. Ricci, S. Zara, B. Mathew, Pharmaceuticals 14, 183 (2021)
Funding
This research received intramural grants from “G. d’Annunzio” University to S.C. (FAR2021).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript. SC and BM conceived the study, provided funding and wrote the paper; S.Z. and A.R. performed the biological evaluation; RFAA, SD, AV, FSK, RAA, KTK and MAA synthesized and characterized the compounds.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azeez, R.F.A., Zara, S., Ricci, A. et al. Integrating N-alkyl amide in the chalcone framework: synthesis and evaluation of its anti-proliferative potential against AGS cancer cell line. Res Chem Intermed 49, 203–220 (2023). https://doi.org/10.1007/s11164-022-04864-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04864-w