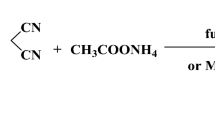Abstract
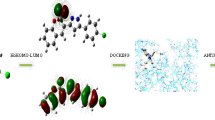
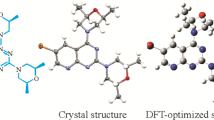
Trifluoromethyl group containing pyrazole-3-carboxamide derivative is synthesized and the structure of the molecule (E3N5PC) has been verified by using FT-IR, 1H NMR, 13C NMR spectroscopic methods and elemental analysis. In order to determine the theoretical characterization, spectroscopic and electronic properties of the title compound, DFT calculations employing B3LYP method with 6-311 + + G(d, p) basis set has been carried out by utilizing Gaussian 09w package program. In addition, VEDA 4xx software has been used to determine the vibrational frequencies of the title molecule with potential energy distribution percentage. Chemical selectivity and reactivity of the drug molecule, which helps to improve the stability of the molecule, are analyzed via NBO calculations. Molecular docking is to find the best fit orientation of the title molecule with the cannabinoid receptor1.










Similar content being viewed by others
References
S. Fustero, M. Sanchez-Rosello, P. Barrio, A. Simon-Fuentes, Chem. Rev. 111, 6984 (2011)
S. Fustero, A. Simon-Fuentes, J.F. Sanz-Cervera, Org. Prep. Proced. Int. 41(4), 253 (2009)
S. Mert, R. Kasimogullari, S. Ok, J. Postdr. Res. 2(4), 64 (2014)
R. Nithyabalaji, H. Krishnan, R. Sribalan, J. Mol. Struct. 1186, 1 (2019)
K. Karrouchi, S. Radi, Y. Ramli, J. Taoufik, Y.N. Mabkhot, F.A. Al-Aizari, M. Ansar, Molecules 23(1), 134 (2018)
F.E. Bennani, L. Doudach, Y. Cherrah, Y. Ramli, K. Karrouchi, M. Ansar, M.E.A. Faouzi, Bioorg. Chem. 97, 103470 (2020)
A. Bekhit, A. Hymete, E.D.A. Bekhit, A. Damtew, Y.H. Aboul-Enein, Mini Rev. Med. Chem. 10(11), 1014 (2010)
M. Abdel-Aziz, G.E.D.A. Abuo-Rahma, A.A. Hassan, Eur. J. Med. Chem. 44(9), 3480 (2009)
D.K. Aneja, P. Lohan, S. Arora, C. Sharma, K.R. Aneja, O. Prakash, Org. Med. Chem. Lett. 1(1), 15 (2011)
F.K. Keter, J. Darkwa, Biometals 25(1), 9 (2012)
S.G. Küçükgüzel, S. Şenkardeş, Eur. J. Med. Chem. 97, 786 (2015)
H. Kumar, D. Saini, S. Jain, N. Jain, Eur. J. Med. Chem. 70, 248 (2013)
G.M. Nitulescu, C. Draghici, O.T. Olaru, Int. J. Mol. Sci. 14(11), 21805 (2013)
P.D. Sauzem, P. Machado, M.A. Rubin, G.D.S. Sant’Anna, H.B. Faber et al., Eur. J. Med. Chem. 43(6), 1237 (2008)
D. Secci, A. Bolasco, P. Chimenti, S. Carradori, Curr. Med. Chem. 18(33), 5114 (2011)
S. Mert, R. Kasımoğulları, T. Iça, F. Çolak, A. Altun, S. Ok, Eur. J. Med. Chem. 78, 86 (2014)
A. Bayrakdar, H.H. Kart, S. Elcin, H. Deligoz, M. Karabacak, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 607 (2015)
B. Sathya, S. Karthi, K. Ajaijawahar, M. Prasath, Res. Chem. Intermed. 46(10), 4475 (2020)
M. Govindammal, M. Prasath, S. Kamaraj, B. Sathya, Biocatal. Agric. Biotechnol. 18, 101086 (2019)
B. Sathya, M. Prasath, Res. Chem. Intermed. 47, 2775 (2021)
B. Sathya, M. Prasath, Res. Chem. Intermed. 45, 2135 (2019)
G. Mol, D. Aruldhas, Bull. Pure Appl. Sci. Chem. 37(1), 20 (2018)
J.I. Ahamed, M.F. Valan, K. Pandurengan et al., Res. Chem. Intermed. 47, 759 (2021)
R. Kasımoğulları, H. Duran, A.S. Yağlıoğlu, S. Mert, I. Demirtaş, Chem. Month. 146(10), 1743 (2015)
R. Kasımoğulları, M. Maden, A.S. Yağlıoğlu, S. Mert, I. Demirtaş, Indian J. Chem. 54B, 1134 (2015)
R. Kasımoğulları, M. Bülbül, S. Mert, H. Güleryüz, J. Enzyme Inhib. Med. Chem. 26(2), 231 (2011)
Gaussian09 RA. 1, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, v. Barone, b. Mennucci, ga petersson et al., (gaussian. Inc, Wallingford CT, 2009)
A. Frisch, A. Nielsen, A. Holder, Gauss View Molecular Visualization Program User Manual (Gaussian Inc, Pittsburg, 2001)
M.H. Jamróz, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 114, 220 (2013)
F. Scalambra, S. Rudić, A. Romerosa, Eur. J. Inorg. Chem. 2019(8), 1155 (2019)
D. Lin-Vien, N.B. Colthup, W.G. Fateley, J.G. Grasselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules (Elsevier, 1991)
N.P. Roeges, J. Baas, A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures (Wiley, 1994)
S.E. Krikorian, M. Mahpour, Spectrochim. Acta Part A Mol. Spectrosc. 29(7), 1233 (1973)
T. Miyazawa, J. Mol. Spectrosc. 4(1–6), 168 (1960)
B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, 1998)
T.B. Issa, F. Sayari, H. Ghalla, L. Benhamada, J. Mol. Struct. 1178, 436 (2019)
B. Nie, J. Stutzman, A. Xie, Biophys. J. 88(4), 2833 (2005)
K.-I. Takei, R. Takahashi, T. Noguchi, J. Phys. Chem. B 112(21), 6725 (2008)
N. Issaoui, H. Ghalla, F. Bardak, M. Karabacak, N.A. Dlala et al., J. Mol. Struct. 1130, 659 (2017)
N. Sundaraganesan, K.S. Kumar, C. Meganathan, B.D. Joshua, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 65(5), 1186 (2006)
N. Wu, X. She, D. Yang, X. Wu, F. Su, Y. Chen, J. Mater. Chem. 22(33), 17254 (2012)
V. Aswathy, Y.S. Mary, P. Jojo, C.Y. Panicker, A. Bielenica et al., J. Mol. Struct. 1134, 668 (2017)
Y.S. Mary, V. Aswathy, C.Y. Panicker, A. Bielenica, P. Brzózka et al., RSC Adv. 6(113), 111997 (2016)
Y.S. Mary, C.Y. Panicker, T. Yamuna, M. Siddegowda, H. Yathirajan et al., Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 132, 491 (2014)
V. Baron, M. Cossi, J. Tomasi, J. Chem. Phys. 107(8), 3210 (1997)
H. Sklenar, J. Jäger, Int. J. Quantum Chem. 16(3), 467 (1979)
I. Fleming, Molecular Orbitals and Organic Chemical Reactions (Wiley, 2011)
D. Sajan, L. Joseph, N. Vijayan, M. Karabacak, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 81(1), 85 (2011)
N. Subramanian, N. Sundaraganesan, J. Jayabharathi, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 76(2), 259 (2010)
L.D. Mendelsohn, J. Chem. Inf. Comput. Sci. 44(6), 2225 (2004)
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew et al., J. Comput. Chem. 30(16), 2785 (2009)
S.M.D. Rizvi, S. Shakil, M.A. Haneef, EXCLI J. 12, 831 (2013)
E.F. Pettersen, T.D. Goddard, C.C. Huang, G.S. Couch, D.M. Greenblatt et al., J. Comput. Chem. 25(13), 1605 (2004)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bayrakdar, A., Mert, S., Kasımoğulları, R. et al. Synthesis, spectroscopic (FT-IR and NMR), DFT and molecular docking studies of ethyl 1-(3-nitrophenyl)-5-phenyl-3-((4-(trifluoromethyl)phenyl)carbamoyl)-1H-pyrazole-4-carboxylate. Res Chem Intermed 48, 2087–2109 (2022). https://doi.org/10.1007/s11164-022-04681-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04681-1