Abstract
Nanocrystalline powders of alkali metal titanates were prepared using titanium tetraisopropoxide and alkali metal salts by a sol–gel method. The influences of the kinds of alkali metal salts and heating temperature on the reaction of the titanium precursor compounds with the alkali metal salts, its phase transition, and crystal growth were investigated by thermal analyses, XRD measurement, and SEM observation in order to prepare lithium, sodium, and potassium titanates at relatively low temperatures. The amorphous titania gel or its product, very small titania crystals, as an intermediate phase reacted with the alkali metal salts at around 500–600 °C while it was still highly active and formed alkali metal titanate nanoparticles before forming the stable titania crystals. It is important that the active amorphous titania gels react with the dispersed alkali metal salts at a temperature as low as possible in order to form the alkali metal titanate nanocrystals.






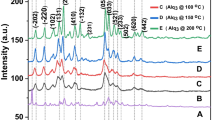
source and using a sodium chloride or b a mixture of sodium chloride and sodium nitrate as the flux at the Na/Ti ratio of 5/6


source and using a sodium chloride or b a mixture of sodium chloride and sodium nitrate as the flux at the Na/Ti ratio of 5/6

source and using potassium chloride as the flux at the K/Ti ratio of 5/1

source and using potassium chloride at the K/Ti ratio of 100/1. K2Ti8O17 (ICDD PDF 41–1100), K2Ti4O9 (ICDD PDF 32–0861)

source and using potassium chloride at the K/Ti ratio of 100/1
Similar content being viewed by others
References
P. Afanasiev, J. Mater. Sci. 41, 1187 (2006)
P. Ponce-Peña, M. Poisot, A. Rodríguez-Pulido, M.A. González-Lozano, Mater. 12, 4132 (2019)
S. Chauque, F.Y. Oliva, G. Lener, O.R. Cámara, J. Solid State Electrochem. 24, 1017 (2020)
S. Moghiminia, H. Farsi, H. Raissi, Electrochim. Acta 132, 512 (2014)
N. Eskandari, G. Nabiyouni, S. Masoumi, D. Ghanbari, Composites B 176, 107343 (2019)
X. Chen, S.S. Mao, Chem. Rev. 107, 2891 (2007)
P. Hartmann, D.K. Lee, B.M. Smarsly, J. Janek, ACS Nano 4, 3147 (2010)
S. Mukherjee, C.D. Quilty, S. Yao, C.A. Stackhouse, L. Wang, K.J. Takeuchi, E.S. Takeuchi, F. Wang, A.C. Marschilok, E. Pomerantseva, J. Mater. Chem. A 8, 18220 (2020)
J. Mosa, M. Aparicio, K. Tadanaga, A. Hayashi, M. Tatsumisago, Electrochim. Acta 149, 293 (2014)
J. Mosa, M. Aparicio, Nanomater. 10, 1369 (2020)
T.K. Saothayanun, T.T. Sirinakorn, M. Ogawa, Inorg. Chem. 59, 4024 (2020)
C.Y. Kang, M. Krajewski, J.Y. Lin, J. Solid State Electrochem. 25, 575 (2021)
G. Tan, X. Hu, S. Wang, X. Yang, Y. Li, Z. Yang, Y. Zhang, Ceram. Int. 46, 27686 (2020)
D. Wang, X. Wu, Y. Zhang, J. Wang, P. Yan, C. Zhang, D. He, Ceram. Int. 40, 3799 (2014)
K. Teshima, H. Inagaki, S. Tanaka, K. Yubuta, M. Hozumi, K. Kohama, T. Shishido, S. Oishi, Cryst. Growth Des. 11, 4401 (2011)
Y. Chiba, D. Koizumi, M. Saito, T. Motohashi, CrystEngComm 21, 3223 (2019)
A.-L. Sauvet, S. Baliteau, C. Lopez, P. Fabry, J. Solid State Chem. 177, 4508 (2004)
J. Ramírez-Salgado, E. Djurado, P. Fabry, J. Eur. Ceram. Soc. 24, 2477 (2004)
S.O. Kang, H.S. Jang, Y.I. Kim, K.B. Kim, M.J. Jung, Mater. Lett. 61, 473 (2007)
L.F. Garay-Rodríguez, L.M. Torres-Martínez, J. Sol-Gel Sci. Technol. 93, 428 (2020)
J. J. Machorro López, A. L. Lázaro, F. J. Rodriguez-Valadez, F. Espejel-Ayala, Environ. Prog. Sustain. Ener. 40, e13475 (2021)
H. Nishikiori, Y. Fukasawa, Y. Yokosuka, T. Fujii, Res. Chem. Intermed. 37, 869 (2011)
J. Pagáčová, A. Plško, I. Papučová, K. Faturíková, A. Prnová, J. Valuchová, D. Ondrušová, J. Therm. Anal. Calorim. 142, 1643 (2020)
F.D. Hardcastle, H. Ishihara, R. Sharma, A.S. Biris, J. Mater. Chem. 21, 633 (2011)
R.D. Shannon, J.A. Pask, J. Am. Ceram. Soc. 48, 391 (1965)
H. Nishikiori, M. Takei, K. Oki, S. Takano, N. Tanaka, T. Fujii, Appl. Catal. B: Environ. 127, 227 (2012)
H. Nishikiori, T. Akaozeki, T. Hizumi, N. Zettsu, K. Teshima, Chem. Lett. 45, 729 (2016)
H. Nishikiori, T. Hizumi, K. Kawamoto, K. Teshima, Res. Chem. Intermed. 44, 7539 (2018)
U. Diebold, Surf. Sci. Rep. 48, 53 (2003)
F. Xiao, G.Q. Jiang, J.Y. Chen, Z.L. Jiang, X.Z. Liu, A. Osaka, X.C. Ma, J. Mater. Sci. 53, 285 (2018)
J.W. Kim, H.G. Lee, Metall. Mater. Trans. B 32, 17 (2001)
S. Yuvaraj, L. Fan-Yuan, C. Tsong-Huei, Y. Chuin-Tih, J. Phys. Chem. B 107, 1044 (2003)
M.J. Geselbracht, L.D. Noailles, L.T. Ngo, J.H. Pikul, Chem. Mater. 16, 1153 (2004)
P. Billik, M. Čaplovičová, Ľ Čaplovič, Mater. Res. Bull. 45, 621 (2010)
H. Yoshida, M. Sato, N. Fukuo, L. Zhang, T. Yoshida, Y. Yamamoto, T. Morikawa, T. Kajino, M. Sakano, T. Sekito, S. Matsumoto, H. Hirata, Catal. Today 303, 296 (2018)
Y. Dessureault, J. Sangster, A.D. Pelton, J. Chem. Ref. Data 19, 1149 (1990)
E. Baché, C. Le Bot, S. Fereres, E. Palomo, Energy Procedia 49, 715 (2014)
P.J.P. Naeyaert, M. Avdeev, N. Sharma, H.B. Yahia, C.D. Lin, Chem. Mater. 26, 7067 (2014)
S. Altin, S. Demirel, E. Oz, E. Altin, C. Hetherington, A. Bayri, S. Avci, CrystEngComm 22, 2483 (2020)
D. Sergeev, E. Yazhenskikh, D. Kobertz, M. Müller, Calphad 65, 42 (2019)
N. Bao, Z. Feng, L. Shen, X. Lu, Cryst. Growth Des. 2, 437 (2002)
J. Cao, A. Wang, H. Yin, L. Shen, M. Ren, S. Han, Y. Shen, L. Yu, T. Jiang, Ind. Eng. Chem. Res. 49, 9128 (2010)
M.A. Siddiqui, V.S. Chandela, A. Azam, Appl. Surf. Sci. 258, 7354 (2012)
H. Yoshida, M. Takeuchi, M. Sato, L. Zhang, T. Teshima, M.G. Chaskar, Catal. Today 232, 158 (2014)
S. Suzuki, K. Teshima, M. Kiyohara, H. Kamikawa, K. Yubuta, T. Shishido, S. Oishi, CrystEngComm 14, 4176 (2012)
D.V. Marinin, G.N. Brown, Waste Manage. 20, 54 (2000)
M.N. Akieh, M. Lahtinen, A. Väisänen, M. Sillanpää, J. Hazardous Mater. 152, 640 (2008)
J. Ryu, S. Kim, H.J. Hong, J. Hong, M. Kim, T. Ryu, I.S. Park, K.S. Chung, J.S. Jang, B.G. Kim, Chem. Eng. J. 304, 503 (2016)
Z. Chen, Y. Wu, Y. Wei, H. Mimura, J. Radioanal, Nucl. Chem. 307, 931 (2016)
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 15K05472 and JP16KK0110.
Funding
Hiromasa Nishikiori was supported by JSPS KAKENHI Grant Numbers 15K05472 and JP16KK0110.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and writing were performed by Hiromasa Nishikiori. Methodological design was also performed by Fumitaka Hayashi. Material preparation, data collection, and analysis were performed by Hiroyoshi Ebara, Hitoshi Takayama, Shinnosuke Adachi, and Naoya Kobayashi. This study was supervised by Katsuya Teshima.
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishikiori, H., Ebara, H., Takayama, H. et al. Formation of alkali metal titanate nanocrystals using titanium alkoxide. Res Chem Intermed 47, 5135–5153 (2021). https://doi.org/10.1007/s11164-021-04581-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04581-w