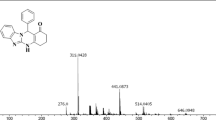
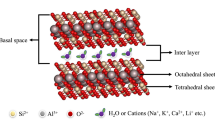
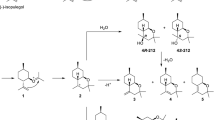
Abstract
One-pot three-component synthesis of twelve different chromeno[2,3-b]indole derivatives were achieved by the condensation of β-naphthol, oxindole and various substituted aldehydes. Two more chromeno[2,3-b]indole derivatives were also synthesized through one-pot two-component condensation of salicylaldehyde with oxindole/chlorooxindole. Both the condensations were achieved by using Keggin-type heteropoly-11-molybdo-1-vanadophosphoric acid, H4[PVMo11O40] supported on montmorillonite K-10 clay for about 10% as catalyst under environmentally benign solvent-free reaction condition. Shorter reaction time, excellent yield of product, sustainability of catalytic material and simple workup procedure under green experimental conditions are the advantages of this protocol.






Similar content being viewed by others
References
M. Curini, G. Cravotto, F. Epifano, G. Giannone, Curr. Med. Chem. 13, 199 (2006)
P.B. Oshiro, B.A. Bregadiolli, L.C. Da Silva-Filho, J. Heterocycl. Chem. 1, 2795 (2020)
P.O. Kennedy, R.D. Thornes (eds.), Coumarins: Biology, Applications and Mode of Action (J. Wiley, Chichester, 1997)
X. Lai, C. Che, ACS Omega 5, 21968 (2020)
P. Biswas, J. Ghosh, C. Bandyopadhyay, Synth. Commun. 46, 759 (2016)
W.J. Houlihan, W.A. Remers, R.K. Brown, Indoles: Part I (Wiley, NY, 1992).
R. J. Sundberg, The Chemistry of Indoles, vol. 18, 1st edn. (Academic Press, NY 1970)
C. Guillonneau, A. Pierre, Y. Charton, N. Guilbaud, L. Kraus-Berthier, S. Leonce, A. Michel, E. Bisagni, G. Atassi, J. Med. Chem. 42, 2191 (1999)
R. Momose, N. Tanaka, J. Fromont, J. Kobayashi, Org. Lett. 15, 2010 (2013)
W. Peng, M. Switalska, L. Wang, Z.W. Mei, Y. Edazawa, C.Q. Pang, I.E.T. El-Sayed, J. Wietrzyk, T. Inokuchi, Eur. J. Med. Chem. 58, 441 (2012)
Y. Nishibayashi, Y. Inada, M. Hidai, S. Uemura, J. Am. Chem. Soc. 124, 7900 (2002)
Y.W. Fang, C.Z. Li, J. Org. Chem. 71, 6427 (2012)
L.W. Ye, X.L. Sun, C.Y. Zhu, Y. Tang, Org. Lett. 8, 3853 (2006)
E.G. Fordos, T. Novak, G. Blasko, I. Fejes, F. Perron-Sierra, M. Nyerges, Heterocycles 87, 2053 (2013)
J. Liu, N. Liu, Y. Yue, Y. Wang, K. Chen, J. Zhang, S. Zhao, K. Zhuo, Chem. Asian J. 12, 401 (2017)
M. Li, Z. Zuo, L. Wen, S. Wang, J. Comb. Chem. 10, 436 (2008)
A. Shaabani, A. Rahmati, E. Farhangi, Tetrahedron Lett. 48, 7291 (2007)
I. Ugi, A. Domling, B. Werner, J. Heterocycl. Chem. 37, 647 (2000)
M. Kumaresan, V. Saravanan, P. Sami, M. Swaminathan, Res. Chem. Intermed. 46, 4193 (2020)
M. Arunachalapandi, S.M. Roopan, Res. Chem. Intermed. (2021).
R. Tayebee, M.F. Abdizadeh, N. Erfaninia, A. Amiri, M. Baghayeri, R.M. Kakhki, B. Maleki, E. Esmaili, Appl Organomet. Chem. 33, e4959 (2019)
F.N. Sani, R. Tayebee, M. Chahkandi, ACS Omega 5, 9999 (2020)
A. Hashemzadeh, M.M. Amini, R. Tayebee, A. Sadeghian, L.J. Durndell, M.A. Isaacs, A. Osatiashtiani, C.M.A. Parlett, A.F. Lee, Mol Catal. 440, 96 (2017)
B. Li, R. Tayebee, E. Esmaeili, M.S. Namaghi, B. Maleki, RSC Adv. 10, 40725 (2020)
R. Tayebee, A. Pejhan, H. Ramshini, B. Maleki, N. Erfaninia, Z. Tabatabaie, E. Esmaeili, Appl Organomet. Chem. 32, 3924 (2017)
R. Tayebee, M.F. Abdizadeh, B. Maleki, E. Shahri, J. Mol. Liq. 241, 447 (2017)
N. Erfaninia, R. Tayebee, E.L. Foletto, M.M. Amini, M. Dusek, F.M. Zonoz, Appl Organomet. Chem. 32, e4047 (2017)
N.M. Ghohe, R. Tayebee, M.M. Amini, A. Osatiashtiani, M.A. Isaacs, A.F. Lee, Tetrahedron 73, 5862 (2017)
Z. Chen, J. Gu, W. Su, J. Chem. Res. 37, 327 (2013)
K. Selvakumar, T. Shanmugaprabha, R. Annapoorani, P. Sami, Synth. Commun. 47, 913 (2017)
K. Selvakumar, T. Shanmugaprabha, M. Kumaresan, P. Sami, Synth. Commun. 47, 2115 (2017)
K. Selvakumar, T. Shanmugaprabha, M. Kumaresan, P. Sami, Synth. Commun. 48, 223 (2018)
M. Kumaresan, V. Karthika, K. Selvakumar, P. Sami, Synth. Commun. 49, 2856 (2019)
G.B. Kauffman, P.F. Vartanian, J. Chem. Educ. 47, 212 (1970)
G.A. Tsigdinos, C.J. Hallada, Inorg. Chem. 7, 437 (1968)
M. Nehate, V.V. Bokade, Appl. Clay Sci. 44, 255 (2009)
S. Boudjema, E. Vispe, A. Choukchou-Braham, J.A. Mayoral, R. Bachir, J.M. Fraile, RSC Adv. 5, 6853 (2015)
M. Kumaresan, K. Selvakumar, P. Sami, Mater. Today: Proc. 14, 12437 (2017)
Acknowledgements
The authors thank the Managing Board authorities of Virudhunagar Hindu Nadars’ Senthikumara Nadar College (Autonomous), Virudhunagar 626001, Tamil Nadu, India, for providing infra-structural and research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antony Muthu, P., Murugan, K., Meenakshisundaram, S. et al. Keggin-type heteropoly-11-molybdo-1-vanadophosphoric acid supported montmorillonite K-10 clay-catalysed one-pot multi-component synthesis of chromeno[2,3-b]indoles. Res Chem Intermed 47, 3583–3595 (2021). https://doi.org/10.1007/s11164-021-04483-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04483-x