Abstract
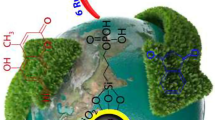
In this work, 1,3,5,7-tetraazaadamantan-1-ium chloride (AIL) functionalized silica-coated calcium oxide hybrid nanocatalyst (CaO@SiO2@AIL) as a novel, efficient, green and recyclable heterogeneous ionic liquid catalyst was synthesized. Catalytic activity of the CaO@SiO2@AIL hybrid nanoparticles was investigated for synthesis of the pharmaceutically valuable 2-imino-2H-chromene and dihydropyrano[c]chromene derivatives. A wide range of amines and aromatic aldehydes containing either electron-withdrawing or electron-donating substituent were examined using optimized conditions to produce the desired products. 2-Imino-2H-chromenes were synthesized under solvent-free condition, and dihydropyrano[c]chromenes were prepared in aqueous medium as green conditions within short reaction times, high yields and using easy workup procedures. Structure confirmation and surface properties of the core/shell hybrid nanoparticles were considered via Fourier transform infrared spectroscopy, X-ray powder diffraction, scanning electron microscope, energy-dispersive X-ray spectroscopy, WDS map scan, thermogravimetric and elemental analyses. This IL-supported heterogeneous nanocatalyst can be reused at least six times without considerable loss of its performance.













Similar content being viewed by others
References
A. Mobinikhaledi, S. Asadbegi, M.A. Bodaghifard, Synth. Commun. 46, 1605 (2016)
G.M. Ziarani, R. Moradi, T. Ahmadi, N. Lashgari, RSC Adv. 8, 12069 (2018)
D. Azarifar, R. Nejat-Yami, F. Sameri, Z. Akrami, Lett. Org. Chem. 9, 435 (2012)
M. Zendehdel, M.A. Bodaghifard, H. Behyar, Z. Mortezaei, Microporous Mesoporous Mater. 266, 83 (2018)
A. Jooya, A. Davoodnia, M. Fattahi, N. Tavakoli-Hoseini, Org. Prep. Proced. Int. 50, 565 (2018)
A. Mobinikhaledi, H. Moghanian, Z. Souri, Lett. Org. Chem. 11, 432 (2014)
A. Mobinikhaledi, A. Yazdanipour, M. Ghashang, Green Process. Synth. 5, 289 (2016)
L. Nazemi Nasirmahale, O. Goli Jolodar, F. Shirini, H. Tajik, Polycycl. Aromat. Compd. (2019). https://doi.org/10.1080/10406638.2019.1576748
Q. Ren, W.Y. Siau, Z. Du, K. Zhang, J. Wang, Chem. Eur. J. 17, 7781 (2011)
M.A. Bodaghifard, M. Solimannejad, S. Asadbegi, S. Dolatabadifarahani, Res. Chem. Intermed. 42, 1165 (2016)
A. Sakurai, Y. Motomura, H. Midorikawa, J. Org. Chem. 37, 1523 (1972)
F. Areias, M. Costa, M. Castro, J. Brea, E. Gregori-Puigjané, M.F. Proença, J. Mestres, M.I. Loza, Eur. J. Med. Chem. 54, 303 (2012)
H. Kiyani, M.D. Daroonkala, Bull. Chem. Soc. Ethiop. 29, 449 (2015)
D. Guo, T. Chen, D. Ye, J. Xu, H. Jiang, K. Chen, H. Wang, H. Liu, Org. Lett. 13, 2884 (2011)
S. Kovalenko, I. Bylov, K. Sytnik, V. Chernykh, Y. Bilokin, Molecules 5, 1146 (2000)
X.L. Shi, X. Xing, H. Lin, W. Zhang, Adv. Synth. Catal. 356, 2349 (2014)
Y. Wang, H. Ye, G. Zuo, J. Luo, J. Mol. Liq. 212, 418 (2015)
Z. Vafajoo, H. Veisi, M.T. Maghsoodlou, H. Ahmadian, C. R. Chim. 17, 301 (2014)
J. Safaei-Ghomi, F. Eshteghal, H. Shahbazi-Alavi, Polycycl. Aromat. Compd. 1, 2 (2017)
M. Cheraghipoor, M.T. Maghsoodlou, M.R. Faghihi, Polycycl. Aromat. Compd. 1 (2019)
A. Kakanejadifard, F. Azarbani, N. Khosravani, B. Notash, J. Mol. Liq. 221, 211 (2016)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7, 890 (2010)
H. Mehrabi, M. Kazemi-Mireki, Chin. Chem. Lett. 22, 1419 (2011)
A.T. Khan, M. Lal, S. Ali, M.M. Khan, Tetrahedron Lett. 52, 5327 (2011)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
R. Mohammadipour, A. Bamoniri, B. Mirjalili, Sci. Iran. 27, 1216 (2020)
N.N. Pesyan, G.R. Bardajee, E. Kashani, M. Mohammadi, H. Batmani, Res. Chem. Intermed. 46, 347 (2020)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H.R. Khavasi, Chem. Comm. 47, 9230 (2011)
M. Bodaghifard, A. Mobinikhaledi, S. Asadbegi, Appl. Organomet. Chem. 31, e3557 (2017)
S. Asadbegi, M.A. Bodaghifard, A. Mobinikhaledi, Res. Chem. Intermed. 46, 1629 (2020)
M.A. Nasseri, M. Sadeghzadeh, J. Chem. Sci. 125, 537 (2013)
E. Mosaddegh, A. Hassankhani, Chin. J. Catal. 35, 351 (2014)
A.O. Etim, P. Musonge, A.C. Eloka-Eboka, Biofuel Bioprod. Biorefin. 14, 620 (2020)
C. Harripersadth, P. Musonge, Y.M. Isa, M.G. Morales, A. Sayago, S. Afr. J. Chem. Eng. 34, 142–150 (2020)
C.T. Yamashita, J. So, M. Fuji, J. Ceram. Soc. Jpn. 124, 55 (2016)
R. Ghorbani-Vaghei, N. Sarmast, Res. Chem. Intermed. 44, 4483 (2018)
K. Lu, J. Zhao, Chem. Eng. J. 160, 788 (2010)
O. Polezhaeva, N. Yaroshinskaya, V. Ivanov, Inorg. Mater. 44, 51 (2008)
P.R. Pandit, M. Fulekar, J. Environ. Manag. 198, 319 (2017)
W. Stöber, A. Fink, E. Bohn, J. Colloid Interface Sci. 26, 62 (1968)
A. Mobinikhaledi, H. Moghanian, M. Ghanbari, Appl. Organomet. Chem. 32, e4108 (2018)
T.A. Hill, A. Mariana, C.P. Gordon, L.R. Odell, M.J. Robertson, A.B. McGeachie, N. Chau, J.A. Daniel, N.N. Gorgani, P.J. Robinson, J. Med. Chem. 53, 4094 (2010)
A. Filipe, C. Marta, C. Marian, B. José, G. Ellisabet, P. Fernanda, M. Jordi, I. Maria, Eur. J. Med. Chem. 54, 303 (2012)
N. Edraki, A. Iraji, O. Firuzi, Y. Fattahi, M. Mahdavi, A. Foroumadi, M. Khoshneviszadeh, A. Shafiee, R. Miri, J. Iran. Chem. Soc. 13, 2163 (2016)
M.H. Helal, E.-H. Ali, A. Gameel, A.A. Ali, Y.A. Ammar, J. Chem. Res. 34, 465 (2010)
Z. Karimi-Jaberi, M.S. Moaddeli, M. Setoodehkhah, M.R. Nazarifar, Res. Chem. Intermed. 42, 4641 (2016)
M. Abaszadeh, M. Seifi, Res. Chem. Intermed. 41, 7715 (2015)
S.M. Habibi-Khorassani, M.T. Maghsoodlou, M. Shahraki, S. Talaiefar, M.A. Kazemian, J. Aboonajmi, Res. Chem. Intermed. 41, 5821 (2015)
P. Rezaee, S. Elahi, J. Davarpanah, J. Porous Mater. 26, 541 (2019)
M. Safaiee, M.A. Zolfigol, F. Afsharnadery, S. Baghery, RSC Adv. 5, 102340 (2015)
D.-Q. Shi, N. Wu, Q.-Y. Zhuang, J. Chem. Res. 2008, 542 (2008)
H.-J. Wang, J. Lu, Z.-H. Zhang, Monatsh. Chem. 141, 1107 (2010)
J.M. Khurana, K. Vij, J. Chem. Sci. 124, 907 (2012)
A. Montaghami, N. Montazeri, Orient. J. Chem. 30, 1361 (2014)
Acknowledgements
We gratefully acknowledge the financial support of this work by the research council of Arak University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11164_2020_4295_MOESM1_ESM.docx
Supporting information includes detailed information about the experimental procedures, FT-IR, 1HNMR, 13CNMR spectra and elemental analysis of some of new compounds. (DOCX 1760 kb)
Rights and permissions
About this article
Cite this article
Sameri, F., Mobinikhaledi, A. & Bodaghifard, M.A. High-efficient synthesis of 2-imino-2H-chromenes and dihydropyrano[c]chromenes using novel and green catalyst (CaO@SiO2@AIL). Res Chem Intermed 47, 723–741 (2021). https://doi.org/10.1007/s11164-020-04295-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04295-5