Abstract
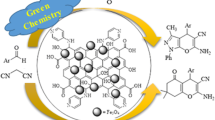
Polystyrene (PS)-coated magnetic graphene oxide (GO-Fe3O4) nanocomposite has been prepared and characterized by FT-IR, TEM and VSM techniques, and its catalytic activity has been evaluated in the multi-component synthesis of two series of indene derivatives. The three-component condensation of ninhydrin/isatin, malononitrile and active methylene-containing compounds and also the four-component condensation of ninhydrin, o-phenylenediamines, malononitrile and active methylene-containing compounds were satisfactorily performed in the presence of PS@GO-Fe3O4 magnetic nanocomposite, and corresponding 2′-aminospiro[indene-2,4′-pyran]-3′-carbonitriles, 2′-aminospiro[indoline-3, 4′-pyran]-3′-carbonitriles and 2′-aminospiro[indeno[1,2-b]quinoxaline-11,4′-pyrane]-3′-carbonitriles were obtained in good-to-excellent yields, respectively. Very shorter reaction times than the reported methods, easy workup procedure and easy separation and reusability of the catalyst are noteworthy advantages of the current method.








Similar content being viewed by others
References
M.J. Kukla, H.J. Breslin, C.J. Diamond, P.P. Grous, C.Y. Ho, M. Miranda, J.D. Rodgers, R.G. Sherrill, E. Clercq, R. Pauwels, J. Med. Chem. 34, 3187 (1991)
J.T.C. Wojtyk, E. Buncel, P.M. Kazmaier, Chem. Commun. 1703 (1998)
B.M. Trost, Science 254, 1471 (1991)
A. Hasaninejad, A. Zare, M. Shekouhy, J. Ameri Rad, J. Comb. Chem. 12, 844 (2010)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, F. Abi, A. Zare, H. Kaveh, Tetrahedron 69, 212 (2013)
A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, A. Zare, A.R. Pourali, R.J. Ayazi-Nasrabadi, Synlett 25, 193 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, M.J. Daraei, Synlett 25, 1173 (2014)
H.I. El-Subbagh, S.M. Abu-Zaid, M.A. Mahran, F.A. Badria, A.M. Al-Obaid, J. Med. Chem. 43, 2915 (2000)
R. Hekmatshoar, S. Majedi, K. Bakhtiari, Catal. Commun. 9, 307 (2008)
K. Singh, J. Singh, H. Singh, Tetrahedron 52, 14273 (1996)
M. Darbarwar, V. Sundaramurthy, Synthesis 337 (1982)
T.-S. Jin, R.-Q. Zhao, T.-S. Li, Arkivoc 11, 176 (2006)
M. Veverka, E. Kraľovičová, Collect. Czechoslov. Chem. Commun. 55, 833 (1990)
A. Darehkordi, Z. Karimi-Taleghani, O.A. Pouralimardan, J. Iran. Chem. Soc. 11, 623(2014)
R.C. Gadwood, B.V. Kamdar, L.A.C. Dubray, M.L. Wolfe, M.P. Smith, W. Watt, J. Med. Chem. 36, 1480 (1993)
M.M. Badran, A.A. Moneer, H.M. Refaat, A.A. El-Malah, J. Chin. Chem. Soc. 54, 469 (2007)
R. Sarges, H.R. Howard, R.G. Browne, L.A. Lebel, P.A. Seymour, B.K. Koe, J. Med. Chem. 33, 2240 (1990)
S.T. Hazeldine, L. Polin, J. Kushner, K. White, N.M. Bouregeois, B. Crantz, J. Med. Chem. 45, 3130 (2002)
X. Li, L. Yang, Ch. Peng, X. Xie, H.-J. Leng, B. Wang, Zh-W Tang, G. He, L. Ouyang, W. Huang, B. Han, Chem. Commun. 49, 8692 (2013)
X. Xie, L. Xiang, Ch. Peng, B. Han, Chem. Rec. 19, 1 (2019)
A. Ding, M. Meazza, H. Guo, J.W. Yang, R. Rios, Chem. Soc. Rev. 47, 5946 (2018)
X. Xie, W. Huang, Ch. Peng, B. Han, Adv. Synth. Catal. 360, 194 (2018)
A. Korte, J. Legros, C. Bolm, Synlett 2397 (2004)
J.A. Makawana, M.P. Patel, R.G. Patel, Arch. Pharm. 345, 314 (2012)
A. Hasaninejad, N. Golzar, A. Zare, J. Heterocycl. Chem. 50, 608 (2013)
A. Hasaninejad, N. Golzar, M. Shekouhy, A. Zare, Helvetica 94, 2289 (2011)
E. Soleimani, M. Hariri, P. Saei, ChemistrySelect 16, 773 (2013)
F. Chen, J. Zheng, M. Huang, Y. Li, Chem. Sci. 41, 5545 (2015)
X. Wu, P. Kiu, Macromol. Res. 18, 1008 (2010)
R. Sengupta, M. Bhattacharya, S. Bandyopadhyay, A.K. Bhowmick, Prog. Polym. Sci. 36, 638 (2011)
K.S. Kim, Y. Zhao, H. Jang, S.Y. Lee, J.M. Kim, K.S. Kim, J.H. Ahn, P. Kim, J.Y. Choi, B.H. Hong, Nature 457, 706 (2009)
J.T. Robinson, F.K. Perkins, E.S. Snow, Z.Q. Wei, P.E. Sheehan, Nano Lett. 8, 3137 (2008)
M.D. Stoller, S.J. Park, Y.W. Zhu, J.H. An, R.S. Ruff, Nano Lett. 8, 3498 (2008)
G. Eda, G. Fanchini, M. Chhowalla, Nat. Nanotechnol. 3, 270 (2008)
E. Yoo, J. Kim, E. Hosono, H. Zhou, T. Kudo, I. Honma, Nano Lett. 8, 2277 (2008)
R. Muszynski, B. Seger, P.V. Kamat, J. Phys. Chem. C 112, 5263 (2008)
B. Zahed, H. Hosseini-Monfared, Appl. Surf. Sci. 328, 536 (2015)
Y. Li, Y. Cao, J. Xie, D. Jia, H. Qin, Z. Liang, Catal. Commun. 58, 21 (2015)
N. Hashim, Z. Muda, M.Z. Hussein, I.M. Isa, A. Mohamed, A. Kamari, S.A. Bakar, M. Mamat, A.M. Jaafar, J. Mater. Environ. Sci. 7, 3225 (2016)
F. Kim, L.J. Cote, J. Huang, Adv. Mater. 22, 1954 (2010)
M.Z. Kasaee, E. Motamedi, M. Majidi, Chem. Eng. J. 172, 540 (2011)
Ph Biehl, M. von der Lühe, S. Dutz, F.H. Schacher, Polymers 10, 91 (2018)
S.F. Hojati, A.H. Amiri, N. MoeiniEghbali, S. Mohamadi, Appl. Organomet. Chem. 32, e4235 (2018)
S.F. Hojati, A.H. Amiri, S. Mohamadi, N. MoeiniEghbali, Res. Chem. Intermed. 44, 2275 (2018)
S.F. Hojati, A.H. Amiri, H. Raouf, Appl. Organomet. Chem. 31, e3595 (2017)
S.F. Hojati, N. MoeiniEghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018)
S.F. Hojati, M. Moosavifar, T. Ghorbanipoor, CR Chem. 20, 520 (2017)
I. Mohammadpoor-Baltork, A.R. Khosropour, S.F. Hojati, Catal. Commun. 8, 200 (2007)
I. Mohammadpoor-Baltork, A.R. Khosropour, S.F. Hojati, Catal. Commun. 8, 1865 (2007)
D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L.B. Alemany, W. Lu, J.M. Tour, ACS Nano 4, 4806 (2010)
P. Saluja, K. Aggarwal, J.M. Khurana, Synth. Commun. 43, 3239 (2013)
H.R. Safaei, M. Shekouhy, S. Rahmanpur, A. Shirinfeshan, Green Chem. 14, 1696 (2012)
P. Nazari, A. Bazi, S.A. Ayatollahi, H. Dolati, S.M. Mahdavi, L. Rafighdoost, M. Amirmostofian, Iran. J. Pharm. Res. 16, 943 (2017)
E. Soleimani, M. Hariri, P. Saei, CR Chim. 16, 773 (2013)
Acknowledgements
The authors thank Hakim Sabzevari University for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hojati, S.F., Amiri, A. & Mahamed, M. Polystyrene@graphene oxide-Fe3O4 as a novel and magnetically recyclable nanocatalyst for the efficient multi-component synthesis of spiro indene derivatives. Res Chem Intermed 46, 1091–1107 (2020). https://doi.org/10.1007/s11164-019-04021-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04021-w