Abstract
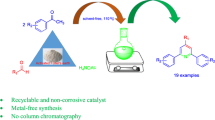
An efficient protocol to synthesize a series of new 1,4-dihydropyridine derivatives under mild conditions is developed. The catalyst consists of iron loaded on zirconia (Fe2O3/ZrO2) and is reusable for up to six cycles. Excellent yields (92–98%) were obtained under room-temperature conditions in a short time (~ 20 min) with ethanol as solvent. Different wt% of catalyst material was prepared by simple wet-impregnation technique, and materials were characterised by powder-XRD, TEM, SEM, BET and FT-IR techniques. The structures of the new 1,4-dihydropyridine derivatives were confirmed employing various spectroscopic techniques. Facile preparation and avoidance of any column separation are the main advantages of this protocol.
Graphical abstract














Similar content being viewed by others
References
B.H. Rotstein, S. Zaretsky, V. Rai, A.K. Yudin, Chem. Rev. 114, 8323 (2014)
A. Domling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
S.V.H.S. Bhaskaruni, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Arab. J. Chem. (2017). https://doi.org/10.1016/j.arabjc.2017.09.016
R.A. Sheldon, Chem. Soc. Rev. 41, 1437 (2012)
R. Pagadala, S. Maddila, S. Rana, S.B. Jonnalagadda, RSC Adv. 4, 6602 (2014)
S.H.S. Bhaskaruni, S. Maddila, W.E. van Zyl, S.B. Jonnalagadda, RSC Adv. 8, 16336 (2018)
C.J. Védrine, Catal. 7, 341 (2017)
S.V.H.S. Bhaskaruni, K.K. Gangu, S. Maddila, S.B. Jonnalagadda, Chem. Rec. (2018). https://doi.org/10.1002/tcr.201800077
V. Menon, V. Popa, C. Contescu, J.A. Schwarz, Rev. Roum. Chim. 43, 393–397 (1998)
S. Kouva, K. Honkala, L. Lefferts, J. Kanervo, Catal. Sci. Technol. 5, 3473 (2015)
Y. Zhao, W. Li, M. Zhang, K. Tao, Catal. Commun. 3, 239 (2002)
V. Kozell, T. Giannoni, M. Nocchetti, R. Vivani, O. Piermatti, L. Vaccaro, Catal. 7, 186 (2017)
M. Sharbatdaran, F. Farzaneh, M.M. Larijani, J. Mol. Catal. A Chem. 382, 79 (2014)
T. Okachi, N. Murai, M. Onaka, Org. Lett. 5, 85 (2003)
M.B. Gawande, R.K. Pandey, R.V. Jayaram, Catal. Sci. Technol. 2, 1113 (2012)
S.V.H.S. Bhaskaruni, S. Maddila, W.E. van Zyl, S.B. Jonnalagadda, Catal. Commun. 100, 24 (2017)
I. Bauer, H.-J. Knölker, Chem. Rev. 115, 3170 (2015)
J.I. Padrón, V.S. Martín, in Iron Catalysis-Fundamentals and Applications, ed. by B. Plietker (Springer, Berlin, 2011), pp. 1–26
B. Åkermark, M.P.T. Sjögren, Adv. Synth. Catal. 349, 2641 (2007)
R.N. Naumov, M. Itazaki, M. Kamitani, H. Nakazawa, J. Am. Chem. Soc. 134, 804 (2012)
N.S. Shaikh, K. Junge, M. Beller, Org. Lett. 9, 5429 (2007)
A. Guđmundsson, K.P.J. Gustafson, B.K. Mai, B. Yang, F. Himo, J.-E. Bäckvall, ACS Catal. 8, 12 (2018)
C. Cassani, G. Bergonzini, C.-J. Wallentin, ACS Catal. 6, 1640 (2016)
J. Hu, V.V. Galvita, H. Poelman, C. Detavernier, G.B. Marin, J. CO2 Util. 17, 20 (2017)
K.-S. Kang, C.-H. Kim, K.-K. Bae, W.-C. Cho, S.-U. Jeong, Y.-J. Lee, C.-S. Park, Chem. Eng. Res. Des. 92, 2584 (2014)
J.-P. Wan, Y. Liu, RSC Adv. 2, 9763 (2012)
M.M. Khan, R. Yousuf, S. Khan, Shafiullah, RSC Adv. 5, 57883 (2015)
M.M. Khan, S. Khan, Saigal, S. Iqbal, RSC Adv. 6, 42045 (2016)
R.N. Goto, L.M. Sobral, L.O. Sousa, C.B. Garcia, N.P. Lopes, J. Marín-Prida, E. Ochoa-Rodríguez, Y. Verdecia-Reyes, G.L. Pardo-Andreu, C. Curti, A.M. Leopoldino, Eur. J. Pharmacol. 819, 198 (2018)
N.C. Desai, A.R. Trivedi, H.C. Somani, K.A. Bhatt, Chem. Biol. Drug Des. 86, 370 (2014)
J. Safaei-Ghomi, H. Shahbazi-Alavi, A. Ziarati, Res. Chem. Intermed. 43, 91 (2017)
J. Wang, N. Li, R. Qiu, X. Zhang, X. Xu, S.-F. Yin, J. Organomet. Chem. 785, 61 (2015)
D.S. Rekunge, C.K. Khatri, G.U. Chaturbhuj, Tetrahedron Lett. 58, 1240 (2017)
P. Sharma, M. Gupta, Green Chem. 17, 1100 (2015)
M. Safaiee, B. Ebrahimghasri, M.A. Zolfigol, S. Baghery, A. Khoshnood, D.A. Alonso, N. J. Chem. 42, 12539 (2018)
K. Azizi, J. Azarnia, M. Karimi, E. Yazdani, A. Heydari, Synlett 27, 1810 (2016)
M.M. Khan, Saigal, S. Khan, S. Shareef, M. Danish, Chem. Sel. 3, 6830 (2018)
J. Davarpanah, M. Ghahremani, O. Najafi, J. Mol. Struct. 1177, 525 (2019)
A. Maleki, V. Eskandarpour, J. Rahimi, N. Hamidi, Carbohydr. Polym. 208, 251 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
B. Maleki, O. Reiser, E. Esmaeilnezhad, H.J. Choi, Polyhedron 162, 129 (2019)
A. Ghorbani-Choghamarani, P. Moradi, B. Tahmasbi, Polyhedron 163, 98 (2019)
S. Bhaskaruni, S. Maddila, W. van Zyl, S. Jonnalagadda, Mol. 23, 1648 (2018)
R. Kumar, N.H. Andhare, A. Shard, Richa, A.K. Sinha, RSC Adv. 4, 19111 (2014)
Q. Li, X. Wang, Y. Yu, Y. Chen, L. Dai, Tetrahedron 72, 8358 (2016)
Acknowledgements
The authors are thankful to the National Research Foundation of South Africa, and University of KwaZulu-Natal, Durban, for financial support and research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhaskaruni, S.V.H.S., Maddila, S., van Zyl, W.E. et al. A green protocol for the synthesis of new 1,4-dihydropyridine derivatives using Fe2O3/ZrO2 as a reusable catalyst. Res Chem Intermed 45, 4555–4572 (2019). https://doi.org/10.1007/s11164-019-03849-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03849-6