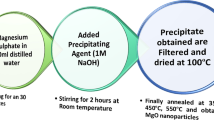
Abstract
A facile one-pot green protocol at room temperature has been devised for the synthesis of novel pyrido[2,3-d]-pyrimidine derivatives catalyzed by CeO2-doped hydroxyapatite (CeO2/HAp) with ethanol as solvent. This highly selective method with excellent yields (89–96%) involves a four-component reaction between malononitrile, substituted aldehyde, dimethylbarbituric acid and ammonium acetate. CeO2/HAp was synthesized and characterized by P-XRD, TGA, TEM and SEM analyses. The structures of the target molecules were confirmed by diverse spectroscopic methods (1H NMR, 15N NMR, 13C NMR, and HRMS). Additional benefits of this eco-friendly approach are the operational simplicity, a stable catalyst with good reusability (at least 6 times), short reaction times (< 45 min) and no need for chromatographic separations. All these features make the proposed method economical and sustainable.
Graphical Abstract







Similar content being viewed by others
References
K. Alfonsi, J. Colberg, P.J. Dunn, T. Fevig, S. Jennings, T.A. Johnson, P. Kleine, C. Knight, M.A. Nagy, D.A. Perry, M. Stefaniak, Green Chem. 10, 31 (2008)
S. Shabalala, S. Maddila, W.E. Van Zyl, S.B. Jonnalagadda, Catal. Commun. 79, 21 (2016)
S. Maddila, S. Rana, R. Pagadala, S. Kankala, S.N. Maddila, S.B. Jonnalagadda, Catal. Commun. 61, 26 (2015)
M.B. Gawande, V.D.B. Bonifacio, R. Luque, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 5522 (2013)
A. Domling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
I.T. Horvath, P.T. Anastas, Chem. Rev. 107, 2169 (2007)
S.N. Maddila, S. Maddila, W.E. Van Zyl, S.B. Jonnalagadda, ChemistryOpen 5, 38 (2016)
S. Maddila, S. Rana, R. Pagadala, S.B. Jonnalagadda, Res. Chem. Intermed. 41, 8269 (2015)
P.J. Walsh, H. Li, C.A.D. Parrodi, Chem. Rev. 107, 2503 (2007)
K.H. Zuo, Y.-P. Zeng, D. Jiang, Mater. Sci. Eng. C 30, 283 (2010)
K. Yamaguchi, K. Mori, T. Mizugaki, K. Ebitani, K. Kaneda, J. Am. Chem. Soc. 122, 7144 (2000)
T. Tsuchida, J. Kubo, T. Yoshioka, S. Sakuma, T. Takeguchi, W. Ueda, J. Catal. 259, 183 (2008)
S. Sebti, R. Tahir, R. Nazih, S. Boulaajaj, Appl. Catal. A Gen. 218, 25 (2001)
B.M. Choudary, C. Sridhar, M.L. Kantam, B. Sreedhar, Tetrahedron Lett. 45, 7319 (2004)
M. Spencer, M. Grynpas, J. Chromat. A. 166, 423 (1978)
Y. Liu, H. Tsunoyama, T. Akita, T. Tsukuda, Chem. Commun. 46, 550 (2010)
R. Stürmer, Angew. Chem. Int. Ed. 38, 3307 (1999)
S.R. Kale, S.S. Kahandal, M.B. Gawande, R.V. Jayaram, RSC Adv. 3, 8184 (2013)
K. Wang, G.J. Kennedy, R.A. Cook, J. Mol. Catal. A Chem. 298, 88 (2009)
S. Koutsopoulos, J. Biomed. Mater. Res. 62, 600 (2002)
M. Melchionna, P. Fornasiero, Mater. Today 17, 349 (2014)
T.A. Farghaly, H.M. Hassaneen, Arch. Pharm. Res. 36, 564 (2013)
F. Herold, J. Kleps, A. Chodkowski, B. Gutkowska, Acta Pol. Pharm. 56, 385 (1999)
A. Banerjee, M.Y. Pawar, S. Patil, P.S. Yadav, P.A. Kadam, V.G. Kattige, D.S. Deshpande, P.V. Pednekar, M.K. Pisat, L.A. Gharat, Bioorg. Med. Chem. Lett. 24, 4838 (2014)
C. Kurumurthy, S.P. Rao, V.B. Swamy, S.G. Kumar, S.P. Rao, B. Narsaiah, L.R. Velatooru, R. Pamanji, V.J. Rao, Eur. J. Med. Chem. 46, 3462 (2011)
M.N. Nasr, M.M. Gineinah, Arch. Pharm. (Weinheim) 335, 289 (2002)
S.P. Satasia, P.N. Kalaria, D.K. Raval, Org. Biomol. Chem. 12, 1751 (2014)
S. Maddila, S.K. Avula, S. Gorle, M. Singh, L. Palakondu, S.B. Jonnalagadda, Lett. Drug Des. Discov. 10, 186 (2013)
D.A. Ibrahim, N.S. Ismail, Eur. J. Med. Chem. 46, 5825 (2011)
Q. Ren, Z. Cui, H. He, Y. Gu, J. Fluor. Chem. 128, 1369 (2007)
A. Agarwal, P.M.S. Chauhan, Tetrahedron Lett. 46, 1345 (2005)
S.E.A. Abdel-Aziz, Synth. Commun. 30, 2223 (2000)
S. Maddila, M. Momin, L. Palakondu, C.V. Rao, J. Saudi Chem. Soc. 20, 173 (2016)
S. Maddila, S.N. Maddila, S.B. Jonnalagadda, L. Palakondu, J. Heterocycl. Chem. 53, 658 (2016)
S. Maddila, J. Valand, H. Bandaru, Y. Kotaiah, L. Palakondu, J. Heterocycl. Chem. 53, 319 (2016)
S. Maddila, K. Naicker, M. Momin, S. Rana, S. Gorle, S.N. Maddila, Y. Kotaiah, M. Singh, S.B. Jonnalagadda, Med. Chem. Res. 25, 283 (2016)
S. Maddila, M. Momin, S. Gorle, L. Palakondu, S.B. Jonnalagadda, J. Chile. Chem. Soc. 60, 2774 (2015)
S. Maddila, R. Pagadala, S.B. Jonnalagadda, Lett. Org. Chem. 10, 693 (2013)
S. Maddila, S. Gorle, M. Singh, L. Palakondu, S.B. Jonnalagadda, Lett. Drug Des. Discov. 10, 977 (2013)
S. Maddila, R. Pagadala, S.B. Jonnalagadda, J. Heterocycl. Chem. 52, 487 (2015)
M.K. Pillai, S. Singh, S.B. Jonnalagadda, Synth. Commun. 40, 3710 (2010)
M.K. Pillai, S. Singh, S.B. Jonnalagadda, Kinet. Catal. 52, 536 (2011)
R. Pagadala, S. Maddila, S. Rana, B. Moodley, N.A. Koorbanally, S.B. Jonnalagadda, RSC Adv. 5, 5627 (2015)
R. Pagadala, S. Maddila, S. Rana, S.B. Jonnalagadda, RSC Adv. 4, 6602 (2014)
Acknowledgement
The authors are grateful to the National Research Foundation (NRF) of South Africa, and the University of KwaZulu-Natal, Durban, for financial support and research facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maddila, S., Gangu, K.K., Maddila, S.N. et al. A viable and efficacious catalyst, CeO2/HAp, for green synthesis of novel pyrido[2,3-d]pyrimidine derivatives. Res Chem Intermed 44, 1397–1409 (2018). https://doi.org/10.1007/s11164-017-3174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3174-2