Abstract
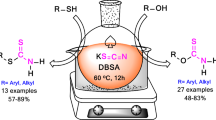
Green synthesis of some novel dithiocarbamate derivatives substituted by aliphatic and aromatic groups as potentially interesting, medicinally important organic compounds via efficient one-pot, catalyst-free reaction is described. In this reaction, dithiocarbamate derivatives are obtained from condensation reaction between primary or secondary amines, carbon disulfide, and alkyl or benzyl halides in one pot and ethanol–aqueous medium. Among aliphatic and aromatic amines, the results generally show that reaction of aliphatic amines with alkyl or benzyl halides led to desired products in highest yields. Also, among aliphatic amines, those which reacted with benzyl halides showed better yields than those that reacted with alkyl halides. Use of environmentally benign solvents is one of the advantages of this procedure. Also, obtaining products in good yield via catalyst-free reaction using a facile, inexpensive, and practical approach can be considered other advantages of this procedure. Target products are very important compounds, because their analogs have been applied in pharmaceutical, chemical, and rubber industries.


Similar content being viewed by others
References
S.L. Cao, Y. Han, C.Z. Yuan, Y. Wang, Z. Xiahou, J. Liao, R.T. Gao, B.B. Mao, B.L. Zhao, Z.F. Li, X. Xu, Eur. J. Med. Chem. 64, 401 (2013)
F. Carta, M. Aggarwal, A. Maresca, A. Scozzafava, R. McKenna, E. Masini, C.T. Supuran, J. Med. Chem. 55, 1721 (2012)
G. Turan-Zitouni, A. Özdemir, K. Güven, Arch. Pharm. Chem. Life Sci. 338, 96 (2005)
N. Lal, S. Jangir, V. Bala, D. Mandalapu, A. Sarswat, L. Kumar, A. Jain, L. Kumar, B. Kushwaha, A.K. Pandey, S. Krishna, T. Rawat, P.K. Shukla, J.P. Maikhuri, M.I. Siddiqi, G. Gupta, V.L. Sharma, Eur. J. Med. Chem. 115, 275 (2016)
G. Brahemi, F.R. Kona, A. Fiasella, D. Buac, J. Soukupova, A. Brancale, A.M. Burger, A.D. Westwell, J. Med. Chem. 53, 2757 (2010)
D. Mehlika, A. Altintop, G. Selen, Y. Özkay, Z.A. Kaplancıkli, Arch. Pharm. Chem. Life Sci. 346, 571 (2013)
S. Levent, U. Cevik, B.N. Saglik, Y. Ozkay, O.D. Can, U.D. Ozkay, U. Uçucu, Phosphorus Sulfur Silicon Relat. Elem. 192, 469 (2017)
W. Yuan, Z. Shang, X. Qiang, Z. Tan, Y. Deng, Res. Chem. Intermed. 40, 787 (2014)
C. Bolzati, M. Cavazza-Ceccato, S. Agostini, F. Refosco, Y. Yamamichi, S. Tokunaga, D. Carta, N. Salvarese, D. Bernardini, G. Bandoli, Bioconjugate Chem. 21, 928 (2010)
S.L. Cao, Y.P. Feng, Y.Y. Jiang, S.Y. Liu, G.Y. Ding, R.T. Li, Bioorg. Med. Chem. Lett. 15, 1915 (2005)
A. Goel, S.J. Majur, R.J. Fattah, T.L. Hartman, J.A. Turpin, M. Huang, W.G. Rice, E. Appella, J.K. Inman, Bioorg. Med. Chem. Lett. 12, 767 (2002)
H. Sudhamani, S.K.T. Basha, S. Muni, C. Reddy, B. Sreedhar, S. Adam, C.N. Raju, Res. Chem. Intermed. 42, 7471 (2016)
Y.G. Suh, Y.S. Lee, K.H. Min, O.H. Park, J.K. Kim, H.S. Seung, S.Y. Seo, B.Y. Lee, Y.H. Nam, K.O. Lee, H.D. Kim, H.G. Park, J. Lee, U.O.J.O. Lim, S.U. Kang, M.J. Kil, J. Koo, S.S. Shin, Y.H. Joo, J.K. Kim, Y.S. Jeong, S.Y. Kim, Y.H. Park, J. Med. Chem. 48, 5823 (2005)
R. Mary-Ann, N.L. Borja, Pharmacotherapy 28, 646 (2008)
D. Chaturvedi, S. Zaidi, Res. Rev. J. Chem. 5, 10 (2016)
H. Hänel, W. Raether, W. Dittmar, Ann. N. Y. Acad. Sci. 544, 329 (1988)
U. Boas, H. Gertz, J.B. Christensen, P.M.H. Heegaard, Tetrahedron Lett. 45, 269 (2004)
A. Ziyaei-Halimehjani, Y. Pourshojaei, M.R. Saidi, Tetrahedron Lett. 50, 32 (2009)
A. Zare, M. Merajoddin, A.R. Moosavi-Zare, M. Zarei, M.H. Beyzavi, M.A. Zolfigol, Res. Chem. Intermed. 42, 2365 (2016)
S.M. Kanan, M.C. Kanan, H.H. Patterson, Res. Chem. Intermed. 32, 871 (2006)
M.J. Hyun, M. Shin, Y.J. Kim, Y.W. Suh, Res. Chem. Intermed. 42, 57 (2016)
W.N. Kun, S. Mlowe, L.D. Nyamen, P.T. Ndifon, M.A. Malik, O.Q. Munro, N. Revaprasadu, Chem. Eur. J. 22, 13127 (2016)
P.J. Nieuwenhuizen, A.W. Ehlers, J.G. Haasnoot, S.R. Janse, J. Reedijk, E.J. Baerends, J. Am. Chem. Soc. 121, 163 (1999)
G. Hogarth, Mini. Rev. Med. Chem. 12, 1202 (2012)
W. Chin-Hsien, Synthesis, 622 (1981)
D. Chaturvedi, S. Ray, Tetrahedron Lett. 47, 1307 (2006)
Y. Ma, J. Xu, Synthesis 44, 2225 (2012)
K. Biswas, S. Ghosh, P. Ghosh, B. Basu, J. Sulfur Chem. 37, 361 (2016)
A. Saha, B.C. Ranu, RSC Adv. 2, 6329 (2012)
N. Azizi, E. Gholibeglo, RSC Adv. 2, 7413 (2012)
Q. Sha, Y.Y. Wei, Org. Biomol. Chem. 11, 5615 (2013)
N.A. Isley, S. Dobarco, B.H. Lipshutz, Green Chem. 16, 1480 (2014)
K. Eskandari, B. Karami, S. Khodabakhshi, J. Chem. Res. 38, 600 (2014)
J.H. Clark, Nat. Chem. 1, 12 (2009)
K. Eskandari, B. Karami, M. Farahi, V. Mouzari, Tetrahedron Lett. 57, 487 (2016)
S. Rahmani-Nezhad, L. Khosravani, M. Saeedi, K. Divsalar, L. Firoozpour, Y. Pourshojaei, Y. Sarrafi, H. Nadri, A. Moradi, M. Mahdavi, A. Shafiee, A. Foroumadi, Synth. Commun. 45, 751 (2015)
Y. Pourshojaei, A. Gouranourimi, S. Hekmat, A. Asadipour, S. Rahmani-Nezhad, A. Moradi, H. Nadri, F.H. Moghadam, S. Emami, A. Foroumadi, A. Shafiee, Eur. J. Med. Chem. 97, 181 (2015)
F. Mehrabi, Y. Pourshojaei, A. Moradi, M. Sharifzadeh, L. Khosravani, R. Sabourian, S. Rahmani-Nezhad, M. Mohammadi-Khanaposhtani, M. Mahdavi, A. Asadipour, H.R. Rahimi, S. Moghimi, A. Foroumadi, Future Med. Chem. 9, 659 (2017)
K. Eskandari, B. Karami, Monatsh. Chem. 147, 2119 (2016)
B. Karami, R. Ferdosian, K. Eskandari, J. Chem. Res. 38, 41 (2014)
B. Karami, K. Eskandari, M. Azizi, Lett. Org. Chem. 10, 722 (2013)
S. Trivedi, Vidya 6, 165 (1963)
R.M. Ottenbrite, J. Chem. Soc. Perkin Trans. 1: Org. Bioorg. Chem. 1972, 88 (1972–1999)
S. Qiang, W. Yun-Yang, Org. Biomol. Chem. 11, 5615 (2013)
C.N. Kapanda, G.G. Muccioli, G. Labar, J.H. Poupaert, D.M. Lambert, J. Med. Chem. 52, 7310 (2009)
M. Toumi, N. Raouafi, K. Boujlel, I. Tapsoba, J.P. Picard, M. Bordeau, Phosphorus Sulfur Silicon Relat. Elem. 182, 2477 (2007)
O. Lieber, J. Org. Chem. 22, 88 (1957)
Acknowledgements
The authors gratefully thank the Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences for supporting this work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asadipour, A., Shams, Z., Eskandari, K. et al. Efficient, straightforward, catalyst-free synthesis of medicinally important S-alkyl/benzyl dithiocarbamates under green conditions. Res Chem Intermed 44, 1295–1304 (2018). https://doi.org/10.1007/s11164-017-3167-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3167-1