Abstract
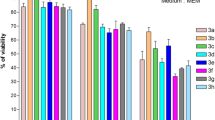
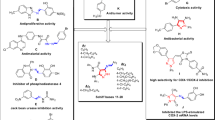
A series of new pyridone 5, 6, 8a–j, hydrazone 7a–j, and thiophene 9–12 derivatives bearing a sulfonamide moiety were synthesized from the starting material 4-chloro-N-(4-(1-(2-(2-cyanoacetyl)hydrazono)ethyl)phenyl) benzenesulfonamide 4. The target compounds were in vitro evaluated for their cytotoxic activity against a human liver cancer cell line (HepG2). Compounds 4 and 8d–j showed higher cytotoxic activity compared to doxorubicin, as a positive control. The radio-sensitizing ability of the promising compounds 4, 8d, and 8h was studied which showed an enhanced cytotoxic activity after combination with γ-radiation. Molecular modeling was performed in CA II/IX mimic active site to predict the binding possibility of the target compounds. All the synthesized compounds showed appropriate fitting with the amino acids in the binding pocket on the basis of their S score data and binding interactions. This binding possibility might contribute at least in part, to their anticancer activity.
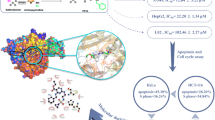
Graphical Abstract
A novel series of sulfonamide derivatives bearing a biologically active pyridone, thiophene, and hydrazone moieties was synthesized and screened for their cytotoxic activity against HepG2 cell line. The most potent compounds in this study 4, 8d, and 8h were evaluated for their radio-sensitizing activity.








Similar content being viewed by others
References
B.A. Chabner, D.L. Longo, Cancer Chemotherapy and Biotherapy: Principles and Practice (Lippincott Williams & Wilkins, Philadelphia, 2011), pp. 761–785
P. Kumar, M. Clark, Clin. Med. 2, 1069 (2002)
R.C. Pandey, M.W. Toussaint, R.M. Stroshane, C.C. Kalita, A.A. Aszalos, A.L. Garretson, T.T. Wei, K.M. Byrne, R.M. Stroshane, R.J. White, J. Antibiot. 34, 1389 (1981)
T. Baladi, V. Abet, S. Piguel, Eur. J. Med. Chem. 105, 220 (2015)
E.A. Motaal, M. Salem, M. Helal, M. El-Gaby, Orient. J. Chem. 31, 875 (2015)
I.W. Cheney, S. Yan, T. Appleby, H. Walker, T. Vo, N. Yao, R. Hamatake, Z. Hong, J.Z. Wu, Bioorg. Med. Chem. Lett. 17, 1679 (2007)
M.D. Wendt, C. Sun, A. Kunzer, D. Sauer, K. Sarris, E. Hoff, L. Yu, D.G. Nettesheim, J. Chen, S. Jin, Bioorg. Med. Chem. Lett. 17, 3122 (2007)
J. Cheng, J.P. Grande, Exp. Biol. Med. 232, 38 (2007)
P.S. Ghosh, K. Manna, U. Banik, M. Das, P. Sarkar, Int. J. Pharm. Sci. 6, 39 (2014)
L.A. Hasvold, W. Wang, S.L. Gwaltney, T.W. Rockway, L.T. Nelson, R.A. Mantei, S.A. Fakhoury, G.M. Sullivan, Q. Li, N.H. Lin, Bioorg. Med. Chem. Lett. 13, 4001 (2003)
R.K. Russell, J.B. Press, R.A. Rampulla, J.J. McNally, R. Falotico, J.A. Keiser, D.A. Bright, A. Tobia, J. Med. Chem. 31, 1786 (1988)
N. Issaeva, P. Bozko, M. Enge, M. Protopopova, L.G. Verhoef, M. Masucci, A. Pramanik, G. Selivanova, Nat. Med. 10, 1321 (2004)
H.L. Lin, H. Zhang, C. Medower, P.F. Hollenberg, W.W. Johnson, Drug Metab. Dispos. 39, 345 (2011)
A. Nerkar, A. Saxena, S. Ghone, A. Thaker, Eur. J. Chem. 6, S97 (2009)
S. Rollas, S.G. Küçükgüzel, Molecules 12, 1910 (2007)
J. Pandey, R. Pal, A. Dwivedi, K. Hajela, Arzneim. Forsch. 52, 39 (2001)
H.Z. Zhang, J. Drewe, B. Tseng, S. Kasibhatla, S.X. Cai, Bioorg. Med. Chem. 12, 3649 (2004)
Z. Liang, D. Zhang, J. Ai, L. Chen, H. Wang, X. Kong, M. Zheng, H. Liu, C. Luo, M. Geng, Bioorg. Med. Chem. Lett. 21, 3749 (2011)
J.L. Buss, B.T. Greene, J. Turner, F.M. Torti, S.V. Torti, Curr. Top. Med. Chem. 4, 1623 (2004)
M.F. Sugrue, Pharmacol. Ther. 43, 91 (1989)
C.T. Supuran, A. Scozzafava, Curr. Med. Chem. Immunol. Endocr. Metab. Agents 1, 61 (2001)
V. Alterio, A. Di Fiore, K. D’Ambrosio, C.T. Supuran, G. De Simone, Chem. Rev. 112, 4421 (2012)
E. Rosatelli, A. Carotti, M. Ceruso, C.T. Supuran, A. Gioiello, Bioorg. Med. Chem. Lett. 24, 3422 (2014)
B.P. Mahon, M.A. Pinard, R. McKenna, Molecules 20, 2323 (2015)
D.A.A. El Ella, M.M. Ghorab, H.I. Heiba, A.M. Soliman, Med. Chem. Res. 21, 2395 (2012)
M.M. Ghorab, D.A.A. El Ella, H.I. Heiba, A.M. Soliman, In-vitro 3, 31 (2011)
M.M. Ghorab, F.A. Ragab, H.I. Heiba, M.G. El-Gazzar, S.S. Zahran, Eur. J. Med. Chem. 92, 682 (2015)
A.B. Pinkerton, T.T. Lee, T.Z. Hoffman, Y. Wang, M. Kahraman, T.G. Cook, D. Severance, T.C. Gahman, S.A. Noble, A.K. Shiau, Bioorg. Med. Chem. Lett. 17, 3562 (2007)
C. Te Chang, Y.L. Yang, U.S. Patent No. 6,046,229 (Google Patents, 2000), p. 6
G.A. Elmegeed, W.K. Khalil, A.A. Raouf, M.M. Abdelhalim, Eur. J. Med. Chem. 43, 763 (2008)
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J.T. Warren, H. Bokesch, S. Kenney, M.R. Boyd, J. Natl. Cancer Inst. 82, 1107 (1990)
L. Rubinstein, R. Shoemaker, K. Paull, R. Simon, S. Tosini, P. Skehan, D. Scudiero, A. Monks, M. Boyd, J. Natl. Cancer Inst. 82, 1113 (1990)
M.M. Ghorab, F.A. Ragab, H.I. Heiba, A.M. Soliman, Eur. J. Med. Chem. 117, 8 (2016)
B.P. McKibben, C.H. Cartwright, A.L. Castelhano, Tetrahedron Lett. 40, 5471 (1999)
R.M. Scrowston, Adv. Heterocycl. Chem. 29, 171 (1981)
K. Tars, D. Vullo, A. Kazaks, J. Leitans, A. Lends, A. Grandane, R. Zalubovskis, A. Scozzafava, C.T. Supuran, J. Med. Chem. 56, 293 (2013)
V. Alterio, M. Hilvo, A. Di Fiore, C.T. Supuran, P. Pan, S. Parkkila, A. Scaloni, J. Pastorek, S. Pastorekova, C. Pedone, Proc. Natl. Acad. Sci. USA 106, 16233 (2009)
J. Leitans, A. Kazaks, A. Balode, J. Ivanova, R. Zalubovskis, C.T. Supuran, K. Tars, J. Med. Chem. 58, 9004 (2015)
C.T. Supuran, World J. Clin. Oncol. 3, 98 (2012)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorab, M.M., Ragab, F.A., Heiba, H.I. et al. Anticancer and radio-sensitizing evaluation of some new sulfonamide derivatives bearing pyridone, thiophene, and hydrazone moieties. Res Chem Intermed 43, 4657–4681 (2017). https://doi.org/10.1007/s11164-017-2903-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2903-x