Abstract
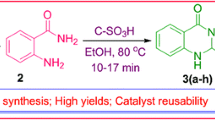
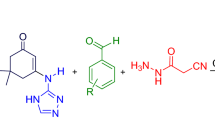
This article describes glycerol mediated eco-friendly approaches for the convenient access of structurally diverse 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ol) and 2-aryl-2,3-dihydroquinazolin-4(1H)-one motifs under catalyst-free conditions. Prominent advantages include clean processes, atom-efficiency, simplicity of the work-up, neutral conditions, low-cost reaction medium, excellent product yield and solvent reusability, in addition to relatively shorter reaction times.
Graphical Abstract






Similar content being viewed by others
References
Y. Zhuang, X. Teng, Y. Wang, P. Liu, G. Li, W. Zhu, Org. Lett. 13, 1130 (2011)
R. Bouley, M. Kumarasiri, Z. Peng, L.H. Otero, W. Song, M.A. Suckow, V.A. Schroeder, W.R. Wolter, E. Lastochkin, N.T. Antunes, H. Pi, S. Vakulenko, J.A. Hermoso, M. Chang, S. Mobashery, J. Am. Chem. Soc. 137, 1738 (2015)
I. Khan, A. Ibrar, W. Ahmed, A. Saeed, Eur. J. Med. Chem. 90, 124 (2015)
P.D. Leeson, B. Springthorpe, Nat. Rev. Drug Discovery 6, 881 (2007)
B.N. Borse, S.R. Shukla, Y.A. Sonawane, Synth. Commun. 42, 412 (2012)
J.F. Wolfe, T.L. Rathman, M.C. Sleevi, J.A. Campbell, T.D. Greenwood, J. Med. Chem. 33, 161 (1990)
J.K. Padia, M. Field, J. Hinton, K. Meecham, J. Pablo, R. Pinnock, B.D. Roth, L. Singh, N.S. Chauhan, B.K. Trivedi, L. Webdale, J. Med. Chem. 41, 1042 (1998)
F. He, P. Li, Y. Gu, G. Li, Green Chem. 11, 1767 (2009)
H.R. Safaei, M. Shekouhy, S. Rahmanpur, A. Shirinfeshan, Green Chem. 14, 1696 (2012)
C.S. Radatz, R.B. Silva, G. Perin, E.J. Lenardão, R.G. Jacob, D. Alves, Tetrahedron Lett. 52, 4132 (2011)
K.U. Sadek, R.A. Mekheimed, A.M.A. Hameed, F. Elnahas, M.H. Elnagdi, Molecules 17, 6011 (2012)
M. Shekouhya, A.M. Sarvestania, S. Khajeha, A.K. Nezhad, RSC Adv. 5, 63705 (2015)
K.P. Nandre, J.K. Salunke, J.P. Nandre, V.S. Patil, A.U. Borse, S.V. Bhosale, Chin. Chem. Lett. 23, 161 (2012)
V.B. Jagrut, D.L. Lingampalle, P.D. Netankar, W.N. Jadhav, Der Pharma Chemica 5, 8 (2013)
F. Nemati, M.M. Hosseini, H.Kiani, J. Saudi Chem. Soc. (2013). doi:10.1016/j.jscs.2013.02.004
M.F. Brana, A. Gradillas, A.G. Ovalles, Bioorg. Med. Chem. 14, 9 (2006)
N. Uramaru, H. Shigematsu, A. Toda, R. Eyanagi, S. Kitamura, S. Ohta, J. Med. Chem. 53, 8727 (2010)
D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. Defelice, M.E. Feigenson, J. Med. Chem. 28, 256 (1985)
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19, 4501 (2009)
D. Singh, D. Singh, J. Indian Chem. Soc. 68, 165 (1991)
J.J. Liu, M.Y. Zhao, X. Zhang, X. Zhao, H.L. Zhu, Mini Rev. Med. Chem. 13, 1957 (2013)
S. Sugiura, S. Ohno, O. Ohtani, K. Izumi, T. Kitamikado, H. Asai, K. Kato, M. Hori, H. Fujimura, J. Med. Chem. 20, 80 (1977)
D. Castagnolo, A.D. Logu, M. Radi, Bioorg. Med. Chem. 16, 8587 (2008)
Y. Liu, G. He, C. Kai, Y. Li, H. Zhu, J. Heterocycl. Chem. 49, 1370 (2012)
M. Londershausen, Pestic. Sci. 48, 269 (1996)
R.C. Maurya, R. Verma, Indian J. Chem., Sect A 36, 596 (1997)
A.D. Garnovskii, A.I. Uraev, V.I. Minkin, Arkivoc 3, 29 (2004)
H. Nakagawa, R. Ohyama, A. Kimata, T. Suzuki, N. Miyata, Bioorg. Med. Chem. Lett. 16, 5939 (2006)
P. Kessler, T. Aybek, G. Neidhart, S. Dogan, D.H. Bremerich, V. Lischke, C. Byhahan, J. Cardiothorac. Vasc. Anesth. 19, 32 (2005)
W.S. Hamama, Synth. Commun. 31, 133 (2001)
T. Ren, S. Liu, G. Li, J. Zhang, J. Guo, W. Li, L. Yang, Spectrochim. Acta, Part A 97, 167 (2012)
M.J. Hour, L.J. Huang, S.C. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel, K.H. Lee, J. Med. Chem. 43, 4479 (2000)
V. Murugan, M. Kulkami, R.M. Anand, E.P. Kumar, B. Suresh, V.M. Reddy, Asian J. Chem. 18, 900 (2006)
H.L. Birch, G.M. Buckley, N. Davies, H.J. Dyke, E.J. Frost, P.J. Gilbert, D.R. Hannah, A.F. Haughan, M.J. Madigan, T. Morgan, W.R. Pitt, A.J. Ratcliffe, N.C. Ray, M.D. Richard, A. Sharpe, A.J. Taylor, J.M. Whitworth, S.C. Williams, Bioorg. Med. Chem. Lett. 15, 5335 (2005)
G. Bonola, R.P. Da, M.J. Magistretti, E. Massarani, I. Setnikar, J. Med. Chem. 11, 1136 (1968)
Rexall Drug Co., U.S. Patent 3257397, 1966
K. Okumura, T. Oine, Y. Yamada, G. Hayashi, M. Nakama, J. Med. Chem. 11, 348 (1968)
E. Cohen, B. Klarberg, J.R. Vaughan, J. Am. Chem. Soc. 81, 5508 (1959)
V. Alagarsamy, V.R. Solomon, M. Murugan, Bioorg. Med. Chem. 15, 4009 (2007)
J. Obniska, K. Shi, Acta Pol. Pharm. 63, 101 (2006)
J.I. Levin, P.I. Chan, T. Bailey, A.S. Katocs, A.M. Venkatesan, Bioorg. Med. Chem. Lett. 4, 1141 (1994)
Instituto De Angeli S.p.A. French Patent M 1893, 1963
Shulton Inc. U.S. Patent 3265697, 1966
N. Hirose, S. Kuriyama, S. Sohda, K. Sakaguchi, H. Yamamoto, Chem. Pharm. Bull. 21, 1005 (1973)
C. Mustazza, A. Borioni, I. Sestili, M. Sbraccia, A. Rodomonte, R. Ferretti, M.R. Giudice, Chem. Pharm. Bull. 54, 611 (2006)
A.N. Gangwal, U.R. Kothawade, A.D. Galande, D.S. Pharande, A.S. Dhake, Indian. J. Heterocycl. Chem. 10, 291 (2001)
A.R. Raghu Ram, R.H. Bahekar, Indian J. Chem 38(B), 434 (1999)
S. Schramm, E. Schmitz, E. Grundemann, J. Prakt. Chem. 326, 279 (1984)
L. He, H. Li, H. Chen, X.F. Wu, RSC Adv. 4, 12065 (2014)
R. Ramesh, A. Lalitha, RSC Adv. 5, 51188 (2015)
R. Ramesh, A. Lalitha, Res. Chem. Intermed. 41, 8009 (2015)
R. Ramesh, S. Maheswari, S. Murugesan, R. Sandhiya, A. Lalitha, Res. Chem. Intermed. 41, 8233 (2015)
R. Ramesh, P. Vadivel, S. Maheswari, A. Lalitha, Res. Chem. Intermed. 42, 7625 (2016)
R. Ramesh, A. Lalitha, ChemistrySelect 1, 2085 (2016)
A. Vafaee, A. Davoodnia, M. Pordel, Res. Chem. Intermed. 41, 8343 (2015)
Z. Zhou, Y. Zhang, Green Chem. Lett. Rev. 7, 18 (2014)
J.S. Ghomi, B.K. Koopaei, H.S. Alavi, RSC Adv. 4, 46106 (2014)
W. Wang, S.X. Wang, X.Y. Qin, J.T. Li, Synth. Commun. 35, 1263 (2005)
M. Zarghani, B. Akhlaghinia, RSC Adv. 5, 87769 (2015)
E. Soleimani, S. Ghorbani, M. Taran, A. Sarvary, C. R. Chimie 15, 955 (2012)
P. Sivaguru, K. Parameswaran, M. Kiruthiga, P. Vadivel, A. Lalitha, J. Iran. Chem. Soc. 12, 95 (2015)
J. Safari, S.G. Ravandi, J. Mol. Catal. A: Chem. 390, 1 (2014)
A.G. Choghamarani, B. Tahmasbi, New J. Chem. 40, 1205 (2016)
M. Rahman, I. Ling, N. Abdulah, R. Hashim, A. Hajra, RSC Adv. 5, 7755 (2015)
N. Kausar, I. Roy, D. Chattopadhyay, A.R. Das, RSC Adv. 2016(6), 22320 (2016)
M. Wang, J. Gao, Z. Song, L. Wang, J. Heterocyclic Chem. 49, 1250 (2012)
M. Sharma, S. Pandey, K. Chauhan, D. Sharma, B. Kumar, M.S. Chauhan, J. Org. Chem. 77, 929 (2012)
J. Chen, W. Su, H. Wu, M. Liub, C. Jin, Green Chem. 9, 972 (2007)
Y.X. Zong, Y. Zhao, W.C. Luo, X.H. Yu, J.K. Wang, Y. Pan, Chin. Chem. Lett. 21, 778 (2010)
V.B. Labade, P.V. Shinde, M.S. Shingare, Tetrahedron Lett. 54, 5778 (2013)
S.G. Zhang, Z.B. Xie, L.S. Liu, M. Liang, Z.G. Le, Chin. Chem. Lett. (2016). doi:10.1016/j.cclet.2016.06.001
K. Revathy, A. Lalitha, J. Iran. Chem. Soc. 12, 2045 (2015)
Acknowledgments
R. R. gratefully acknowledge the DST-Inspire Fellowship, New Delhi, India (No: DST/INSPIRE Fellowship/2012/690) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramesh, R., Nagasundaram, N., Meignanasundar, D. et al. Glycerol assisted eco-friendly strategy for the facile synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ols) and 2-aryl-2,3-dihydroquinazolin-4(1H)-ones under catalyst-free conditions. Res Chem Intermed 43, 1767–1782 (2017). https://doi.org/10.1007/s11164-016-2728-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2728-z