Abstract
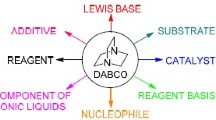
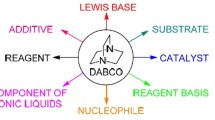
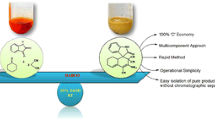
An efficient, three-component strategy for the improved synthesis of a pharmaceutically interesting diverse kind of multi-functionalized benzofurans via one-pot two-step domino protocol with high diastereoselectivity in excellent yields has been established. The synthesis was achieved by reacting phenacyl bromides, N-heterocycles, aromatic aldehydes, and cyclic 1,3-dicarbonyl compounds in the presence of a catalytic amount of DABCO (1,4-diazabicyclo[2.2.2]octane) as an inexpensive, impressive, and readily available catalyst in water under reflux. In this process in total three new bonds (two C–C and one C–O) form in one pot. Short reaction time, excellent yields, no chromatographic purification, and evasion of environmentally hazardous or toxic catalysts and organic solvents in the entire reaction process may make this protocol very useful for academia and industry.


Similar content being viewed by others
References
J. Yu, F. Shi, L.Z. Gong, Acc. Chem. Res. 44, 1156 (2011)
M.S. Singh, G.C. Nandi, T. Chanda, RSC Adv. 3, 14183 (2013)
Z. Xu, M. Ayaz, A.A. Cappelli, C. Hulme, ACS Comb. Sci. 14, 460 (2012)
A.S. Pereteanu, T.J.J. Muller, Org. Biomol. Chem. 11, 5127 (2013)
Q. Gao, P. Zhou, F. Liu, W.J. Hao, C. Yao, B. Jiang, S.J. Tu, Chem. Commun. 51, 9519 (2015)
Q. Gao, W.J. Hao, F. Liu, S.J. Tu, S.L. Wang, G. Li, B. Jiang, Chem. Commun. 52, 900 (2016)
R. Mohebat, A. Yazdani Elah Abadi, M.T. Maghsoodlou, M. Mohammadi, Res. Chem. Intermed. 42, 5915 (2016)
R. Mohebat, A. Yazdani Elah Abadi, M.T. Maghsoodlou, Res. Chem. Intermed. 42, 6039 (2016)
M. Li, H. Cao, Y. Wang, X.L. Lv, L.R. Wen, Org. Lett. 14, 3470 (2012)
J.M. Khurana, A. Chaudhary, A. Lumb, B. Nand, Green Chem. 14, 2321 (2012)
J. McNulty, P. Das, Eur. J. Org. Chem. 24, 4031 (2009)
J. McNulty, P. Das, Tetrahedron Lett. 50, 5737 (2009)
J. McNulty, P. Das, D. McLeod, Chem. Eur. J. 16, 6756 (2010)
S. Narayan, J. Muldoon, M.G. Finn, V.V. Fokin, H.C. Kolb, K.B. Sharpless, Angew. Chem. Int. Ed. 21, 3157 (2005)
P. Das, D. McLeod, J. McNulty, Tetrahedron Lett. 52, 199 (2011)
M. Smith, J. March, March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (Wiley, Hoboken, 2007)
R. Breslow, A fiftty-year perspective on chemistry in Organic Reactions in Water, ed. by M. Lindstrom (Blackwell, Oxford, 2007), pp. 1–28
R. Breslow, D.C. Rideout, J. Am. Chem. Soc. 102, 7816 (1980)
R. Breslow, Acc. Chem. Res. 24, 159 (1991)
G.D. McCallion, Curr. Org. Chem. 3, 67 (1999)
W.S. Sheen, I.L. Tsai, C.M. Teng, I.S. Chen, Phytochemistry 36, 213 (1994)
M. Takasugi, S. Nagao, T. Masamune, A. Shirata, K. Takahashi, Tetrahedron Lett. 19, 797 (1978)
K.M. Zareba, Drugs Today 42, 75 (2006)
G.A. Kraus, I. Kim, Org. Lett. 5, 1191 (2003)
A.A. Deana, G.E. Stokker, E.M. Schultz, R.L. Smith, E.J. Cragoe, H.F. Russo, L.S. Watson, J. Med. Chem. 26, 580 (1983)
E. Wenkert, B.L. Buckwalter, J. Am. Chem. Soc. 94, 4367 (1972)
F.R. Petronijevic, P. Wipf, J. Am. Chem. Soc. 133, 7704 (2011)
J. Boonsompat, A. Padwa, J. Org. Chem. 76, 2753 (2011)
S. Kiren, X. Hong, C.A. Leverett, A. Padwa, Org. Lett. 11, 1233 (2009)
K.C. Nicolaou, P.S. Baran, Y.L. Zhong, K.C. Fong, H.S. Choi, J. Am. Chem. Soc. 124, 2190 (2002)
M. Anary-Abbasinejad, N. Shams, M. Heidari, ARKIVOC ix, 13 (2012)
M.H. Mosslemin, N. Shams, H. Esteghamat, H. Anaraki-Ardakani, Chin. Chem. Lett. 24, 1095 (2013)
M. Anary-Abbasinejad, H.D. Farashah, A. Hassanabadi, H. Anaraki-Ardakani, N. Shams, Synth. Commun. 42, 1877 (2012)
H. Anaraki-Ardakani, M.H. Mosslemin, M. Anary-Abbasinejad, S.H. Mirhosseini, N. Shams, ARKIVOC xi, 343 (2010)
M. Anary-Abbasinejada, N. Shams, A. Hassanabadi, Phosphorus. Sulfur Silicon Relat. Elem. 185, 1823 (2010)
Q.F. Wang, H. Hou, L. Hui, ChG Yan, J. Org. Chem. 74, 7403 (2009)
Acknowledgments
We gratefully acknowledge financial support from the Research Council of the Islamic Azad University of Yazd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golchin, S., Mosslemin, M.H., Yazdani-Elah-Abadi, A. et al. DABCO-catalyzed multi-component domino reactions for the one-pot efficient synthesis of diverse and densely functionalized benzofurans in water. Res Chem Intermed 43, 1735–1749 (2017). https://doi.org/10.1007/s11164-016-2726-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2726-1