Abstract
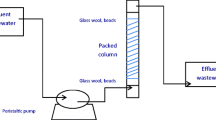
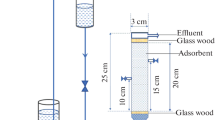
The present study investigates the adsorption capability of raw and biochar forms of Chrysanthemum indicum flowers biomass to remove cobalt ions from aqueous solution in a fixed-bed column. Column adsorption experiments were conducted by varying the bed height (1.0, 2.0, 3.0 cm), flow rate (1.0, 2.5, 5.0 mL min−1) and initial cobalt ion concentration (25, 50, 75 mg L−1) to obtain the experimental breakthrough curves. The adsorption capacity of the raw and biochar forms of C. indicum flowers were found to be 14.84 and 28.34 mg g−1, respectively, for an initial ion concentration of 50 mg L−1 at 1.0 cm bed height and 1.0 mL min−1 flow rate for Co (II) ion adsorption. Adam–Bohart, Thomas and Yoon–Nelson models were applied to the experimental column data to analyze the column performance. The Thomas model was found to best represent the column data with the predicted and experimental uptake capacity values correlating well and with higher R 2 values for all the varying process parameters. Desorption studies revealed the suitability of the adsorbents for repeated use up to four adsorption–desorption cycles without significant loss in its efficiency. It can thus be inferred from the fixed-bed column studies that C. indicum flowers can suitably be used as an effective adsorbent for Co (II) ion removal from aqueous solution on a higher scale.





Similar content being viewed by others
References
P.K. Rai, Crit. Rev. Environ. Sci. Technol. 39, 697 (2009)
H.N. Bhatti, A. Saleem, M.A. Hanif, Desalin. Water Treat. 51, 3335 (2013)
B. Volesky, Z.R. Holan, Biotechnol. Prog. 11, 235 (1995)
S. Rengaraj, S.-H. Moon, Water Res. 36, 1783 (2002)
M. Abbas, S. Kaddour, M. Trari, J. Ind. Eng. Chem. 20, 745 (2014)
C. Caramal, L. Bulgariu, M. Macoveanu, Chem. Bull. “POLITEHNICA” Univ. Timisoara 54, 13 (2009)
L. Tofan, C. Teodosiu, C. Paduraru, R. Wenkert, Appl. Surf. Sci. 285, 33 (2013)
F.-M. Pellera, A. Giannis, D. Kalderis, K. Anastasiadou, R. Stegmann, J.-Y. Wang, E. Gidarakos, J. Environ. Manag. 96, 35 (2012)
B. Volesky, Hydrometallurgy 59, 203 (2001)
D. Sud, G. Mahajan, M. Kaur, Bioresour. Technol. 99, 6017 (2008)
S. Vilvanathan, S. Shanthakumar, Process Saf. Environ. Prot. 96, 98 (2015)
S. Chen, Q. Yue, B. Gao, Q. Li, X. Xu, K. Fu, Bioresour. Technol. 113, 114 (2012)
J. Rodriguez-Carvajal, Match! Phase Identification from Powder Diffraction (2016)
L. Mangaleshwaran, A. Thirulogachandar, V. Rajasekar, C. Muthukumaran, K. Rasappan, J. Taiwan Inst. Chem. Eng. 55, 112 (2015)
S. Sadaf, H.N. Bhatti, J. Taiwan Inst. Chem. Eng. 45, 541 (2014)
K. Vijayaraghavan, J. Jegan, K. Palanivelu, M. Velan, Sep. Purif. Technol. 44, 53 (2005)
X. Xu, B. Gao, X. Tan, X. Zhang, Q. Yue, Y. Wang, Q. Li, Chem. Eng. J. 226, 1 (2013)
R. Han, Y. Wang, X. Zhao, Y. Wang, F. Xie, J. Cheng, M. Tang, Desalination 245, 284 (2009)
J. Cruz-Olivares, C. Pérez-Alonso, C. Barrera-Díaz, F. Ureña-Nuñez, M.C. Chaparro-Mercado, B. Bilyeu, Chem. Eng. J. 228, 21 (2013)
G. Bohart, E. Adams, J. Am. Chem. Soc. 42, 523 (1920)
E.D. Woumfo, J.M. Siéwé, D. Njopwouo, J. Environ. Manag. 151, 450 (2015)
R. Sharma, B. Singh, Bioresour. Technol. 146, 519 (2013)
H.C. Thomas, J. Am. Chem. Soc. 66, 1664 (1944)
J. Samuel, M. Pulimi, M.L. Paul, A. Maurya, N. Chandrasekaran, A. Mukherjee, Bioresour. Technol. 128, 423 (2013)
S. Qaiser, A.R. Saleemi, M. Umar, J. Hazard. Mater. 166, 998 (2009)
K.Y. Foo, L.K. Lee, B.H. Hameed, Bioresour. Technol. 133, 599 (2013)
X. Sun, T. Imai, M. Sekine, T. Higuchi, K. Yamamoto, A. Kanno, S. Nakazono, J. Ind. Eng. Chem. 20, 3623 (2014)
Acknowledgments
The authors would like to thank VIT University for providing Atomic Absorption Spectroscopy (AAS) facility to perform metal concentration analysis and the Department of Chemistry, IIT Madras, Chennai, for providing the facility to carry out the Powder XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilvanathan, S., Shanthakumar, S. Modeling of fixed-bed column studies for removal of cobalt ions from aqueous solution using Chrysanthemum indicum . Res Chem Intermed 43, 229–243 (2017). https://doi.org/10.1007/s11164-016-2617-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2617-5