Abstract
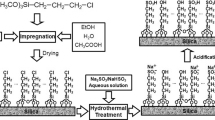
A simple scheme for a mesoporous silica-anchored organotin catalyst was developed for the transesterification of dimethyl carbonate with phenol to diphenyl carbonate. N2-sorption, TEM, UV–Vis, and elemental analysis combined with 29Si and 13C NMR measurements evidenced the formation of mesoporous organic–inorganic hybrid silica with a highly dispersed tetrahedral Sn species. The catalyst exhibited excellent activity and reusability in the transesterification. With a catalyst of 1.0 g, a reaction temperature of 150–180 °C, and a reaction time of 9 h, the phenol conversion and transesterification selectivity reached 51.1 and 99.9 %, respectively. The phenol conversion just decreased from 41.2 to 35.0 % after five runs with 0.5 g of catalyst. The improved stability was attributed to the strong covalent bonding between the organotin and mesoporous silica.






Similar content being viewed by others
References
R. Kanega, H. Ogihara, I. Yamanaka, Res. Chem. Intermediat. 41, 12 (2015)
R. Kanega, I. Yamanaka, Top. Catal. 57, 10–13 (2014)
P. Wang, S. Liu, F. Zhou, B. Yang, A.S. Alshammari, Y. Deng, RSC Adv. 5, 103 (2015)
Z.-H. Fu, Y. Ono, J. Mol. Catal. A Chem. 118, 3 (1997)
K.M. Deshmukh, Z.S. Qureshi, K.P. Dhake, B.M. Bhanage, Catal. Commun. 12, 3 (2010)
A.A.G. Shaikh, S. Sivaram, Ind. Eng. Chem. Res. 31, 4 (1992)
H. Lee, J.Y. Bae, O.-S. Kwon, S.J. Kim, S.D. Lee, H.S. Kim, J. Organomet. Chem. 689, 10 (2004)
F.A. Mercier, M. Biesemans, R. Altmann, R. Willem, R. Pintelon, J. Schoukens, B. Delmond, G. Dumartin, Organometallics 20, 5 (2001)
A.E. Collis, I.T. Horvath, Catal. Sci. Technol. 1, 6 (2011)
C. Camacho-Camacho, M. Biesemans, M. Van Poeck, F.A. Mercier, R. Willem, K. Darriet-Jambert, B. Jousseaume, T. Toupance, U. Schneider, U. Gerigk, Chem. Eur. J. 11, 8 (2005)
V. Pinoie, K. Poelmans, H.E. Miltner, I. Verbruggen, M. Biesemans, G.V. Assche, B. Van Mele, J.C. Martins, R. Willem, Organometallics 26, 27 (2007)
V. Pinoie, M. Biesemans, R. Willem, Organometallics 29, 1 (2009)
A. Corma, H. Garcia, Adv. Synth. Catal. 348, 12–13 (2006)
S.A. Matlin, P.S. Gandham, J. Chem. Soc. Chem. Commun. 798, 12 (1984)
Q.J. Fu, A.M. Steele, S.C. Tsang, Green Chem. 3, 2 (2001)
T. Toupance, L. Renard, B. Jousseaume, C. Olivier, V. Pinoie, I. Verbruggen, R. Willem, Dalton Trans. 42, 26 (2013)
B. Fan, H. Li, W. Fan, J. Zhang, R. Li, Appl. Catal. A 372, 1 (2010)
A. Sayari, B.-H. Han, Y. Yang, J. Chem. Soc., Chem. Commun. 126, 44 (2004)
L. Yoder, Ind. Eng. Chem. 755, 11 (1919)
K. Chaudhari, T. Das, P. Rajmohanan, K. Lazar, S. Sivasanker, A. Chandwadkar, J. Catal. 183, 2 (1999)
B. Fan, J. Zhang, R. Li, W. Fan, Catal. Lett. 121, 3–4 (2008)
P. Yu, J. He, L. Yang, M. Pu, X. Guo, J. Catal. 260, 1 (2008)
A. Bordoloi, S. Sahoo, F. Lefebvre, S. Halligudi, J. Catal. 259, 2 (2008)
F. Adam, H. Osman, K.M. Hello, J. Colloid Interface Sci. 331, 1 (2009)
S. Wang, R. Bai, F. Mei, G. Li, Catal. Commun. 11, 3 (2009)
Z. Li, B. Cheng, K. Su, Y. Gu, P. Xi, M. Guo, J. Mol. Catal. A Chem. 289, 1 (2008)
R. Tang, T. Chen, Y. Chen, Y. Zhang, G. Wang, Chin. J. Catal. 35, 4 (2014)
R. Poller, S. Retout, J. Organomet. Chem. 173, 3 (1979)
Y.T. Kim, E.D. Park, Appl. Catal. A 356, 2 (2009)
Z. Li, Y. Wang, X. Ding, X. Zhao, J. Nat. Gas Chem. 18, 1 (2009)
Acknowledgments
Financial support for this work from the National High Technology Research and Development Program of China (863 program, No. 2013AA031703), and the Science and Technology Support Program of Sichuan Province (No. 2013GZX0135) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, S., Xiao, Z. et al. Mesoporous silica-anchored organotin as heterogeneous catalyst for the transesterification of dimethyl carbonate with phenol. Res Chem Intermed 42, 7213–7222 (2016). https://doi.org/10.1007/s11164-016-2530-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2530-y