Abstract
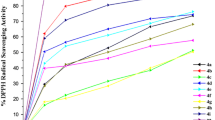
N,N,N′,N′-Tetrachlorobenzene-1,3-disulfonamide and poly(N,N′-dichloro-N-ethyl-benzene-1,3-disulfonamide) are new catalysts promoted by one-pot, facile and four-component synthesis of new substituted 1,4-dihydropyridine derivatives from the reaction of ammonium acetate, aldehydes and various 1,3-dicarbonyl compounds with good to high yields at room temperature under mild conditions. All the synthesized compounds were evaluated for antibacterial and anti-oxidant activities. Antibacterial activity was determined against four Gram-positive and -negative bacteria, and anti-oxidant activity was evaluated by 2,2-diphenyl-1-picrylhydrazyl free radical scavenging. The bioassay results indicated that synthesized 1,4-dihydropyridine derivatives have effective anti-oxidant and antibacterial functions.
Graphical Abstract
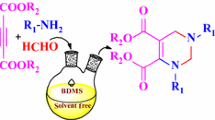
Synthesis and biological evaluation of new series dihydropyridines.




Similar content being viewed by others
References
J.R. Liddell, Nat. Prod. Rep. 19, 773 (2002)
X.Q. Zhu, H.Y. Wang, J.S. Wang, Y.C. Liu, J. Org. Chem. 66, 344 (2001)
Q. Kang, Z.A. Zhao, S.L. You, Org. Lett. 10, 2031 (2008)
M. Rueping, J. Dufour, F.R. Schoepke, Green Chem. 13, 1084 (2011)
R. Lavilla, J. Chem. Soc. Perkin 1, 1141 (2002)
L.M. Yagupolskii, W. Antepohl, F. Artunc, R. Handrock, B.M. Klebanov, I.I. Maletina, B. Marxen, K.I. Petko, U. Quast, A. Vogt, C. Weiss, J. Zibold, S. Herzig, J. Med. Chem. 42, 5266 (1999)
K.C. Majumdar, S.K. Chattopadhyay, Heterocycles in natural product synthesis (Wiley-VCH Verlag & Co. KGaA, Weinheim, 2011), p. 91
L. Guerrier, J. Royer, D.S. Grierson, H.P. Husson, J. Am. Chem. Soc. 105, 7754 (1983)
R. Pena, S. Jimenez-Alonso, G. Feresin, A. Tapia, S. Méndez-Alvarez, F. Machín, A.G. Ravelo, A. Estevez-Braun, J. Org. Chem. 78, 7977 (2013)
C.O. Kappe, W.M.F. Fabian, M.A. Semones, Tetrahedron 53, 2803 (1997)
T. Yamamoto, S. Niwa, S. Ohno, M. Tokumasu, Y. Masuzawa, C. Nakanishi, A. Nakajo, T. Onishi, H. Koganei, S.I. Fujita, T. Takeda, M. Kito, Y. Ono, Y. Saitous, A. Takahara, S. Iwata, M. Shoji, Bioorg. Med. Chem. Lett. 18, 4813 (2008)
G. Swarralatha, G. Prasanthi, N. Sirisha, C. Madhusudhana Chetty, Int. J. ChemTech Res. 3, 75 (2011)
D. Mauzeral, F.H. Westheimer, J. Am. Chem. Soc. 77, 2261 (1955)
R.H. Boecker, F.P. Guengerich, J. Med. Chem. 29, 1596 (1986)
R. Surendra Kumar, A. Idhayadhulla, A. Jamal Abdul Nasser, J. Selvin, J. Serb. Chem. Soc. 76, 1 (2011)
G. Swarnalatha, G. Prasanthi, N. Sirisha, C. Madhusudhana Chetty, Int. J. ChemTech Res. 3, 75 (2011)
R. Surendra Kumar, A. Idhayadhulla, A. Jamal Abdul Nasser, J. Selvin, Eur. J. Med. Chem. 46, 804 (2011)
M. Miri, A. Mehdipour, Bioorg. Med. Chem. 16, 8329 (2008)
J.P. Wan, Y. Liu, RSC Adv. 2, 9763 (2012)
J.P. Wan, Y. Lin, Y. Jing, M. Xu, Y. Liu, Tetrahedron 70, 7874 (2014)
S. Ko, M.N.V. Sastry, C. Lin, C.F. Yao, Tetrahedron Lett. 46, 5771 (2005)
L.M. Wang, J. Sheng, L. Zhang, J.W. Han, Z.Y. Fan, H. Tian, C.T. Qian, Tetrahedron 61, 1539 (2005)
J.L. Donelson, R.A. Gibbs, S.K. De, J. Mol. Catal. A: Chem. 256, 309 (2006)
M. Maheswara, V. Siddaiah, G.L.V. Damu, C.V. Rao, ARKIVOC II, 201 (2006)
M. Maheswara, V. Siddaiah, Y. Koteswara Rao, Y.M. Tzeng, C. Sridhar, J. Mol. Catal. A: Chem. 260, 179 (2006)
H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Aust. J. Chem. 66, 1088 (2013)
S. Tu, Q. Wei, H. Ma, D. Shi, Y. Gao, G. Cui, Synth. Commun. 31, 2657 (2001)
J.C. Legeay, J.J.V. Eynde, J.P. Bazureau, Tetrahedron 61, 12386 (2005)
N.K. Ladani, D.C. Mungra, M.P. Patel, R.G. Patel, Chin. Chem. Lett. 22, 1407 (2011)
S.H.S. Azzam, A. Siddekha, M.A. Pasha, Tetrahedron Lett. 53, 6306 (2012)
S. Balalaie, L. Baoosi, F. Tahoori, F. Rominger, H.R. Bijanzadeh, Tetrahedron 69, 738 (2013)
J. Shun-Jun, J. Zhao-Qin, L. Jun, L. Teck-Peng, Synlett 5, 831 (2004)
S. Pednekar, R. Bhalerao, N. Ghadge, J. Chem. Sci. 125, 615 (2013)
M. Nasr-Esfahani, S. Jafar Hoseini, M. Montazerozohori, R. Mehrabi, H. Nasrabadi, J. Mol. Catal. A: Chem. 382, 99 (2014)
J. Safari, S.H. Banitaba, S. Dehghan Khalili, Chin. J. Catal. 32, 1850 (2011)
A. Kumar, R.A. Maurya, Tetrahedron 63, 1946 (2007)
S.M. Baghbanian, S. Khaksar, S.M. Vahdat, M. Farhang, M. Tajbakhsh, Chin. Chem. Lett. 21, 563 (2010)
S. Cao, S. Zhong, C. Hu, J.P. Wan, C. Wen, Chin. J. Chem. 33, 568 (2015)
R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri, M. Ghavidel, Tetrahedron 67, 1930 (2011)
R. Ghorbani-Vaghei, S. Akbari-Dadamahaleh, Tetrahedron Lett. 50, 1055 (2009)
R. Ghorbani-Vaghei, H. Veisi, Mol. Diversity 14, 249 (2010)
R. Ghorbani-Vaghei, Z. Toghraei-Semiromi, M. Amiri, Mol. Diversity 17, 307 (2013)
R. Ghorbani-Vaghei, M. Amiri, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Ghavidel, Mol. Diversity 17, 251 (2013)
R. Ghorbani-Vaghei, H. Veisi, Synthesis 6, 945 (2009)
H. Veisi, R. Ghorbani-Vaghei, J. Mahmmodi, Bull. Korean Chem. Soc. 32, 3692 (2011)
C.A. Rice-Evans, N.J. Miller, G. Paganga, Free Radic. Biol. Med. 20, 933 (1996)
S. Burt, Int. J. Food Microbiol. 94, 223 (2004)
A. Kumar Dutta, P. Gogoi, R. Borah, RSC Adv. 4, 41287 (2014)
Acknowledgment
We are grateful to Bu-Ali Sina University, Center of Excellence and Development of Chemical Methods (CEDCM) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorbani-Vaghei, R., Malaekehpoor, S.M., Hasanein, P. et al. Synthesis and biological evaluation of new series 1,4-dihydropyridines. Res Chem Intermed 42, 4715–4731 (2016). https://doi.org/10.1007/s11164-015-2310-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2310-0