Abstract
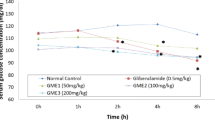
The presently available therapies for type 2 diabetes have not been able to achieve normoglycemic status in a majority of the patients which may be either due to the limitations of the drug itself or its side effects. In an effort to develop potent and safe oral antidiabetic agents, 4-ethyloxychalcone, which was found to be the most potent antiglycating agent in our previous study, has been evaluated for its in vivo hypoglycemic activity using an alloxanized diabetic rat model. The diabetes was induced in rats by injection of intraperitoneal alloxan. However, the oral route was used for the administration of 4-ethyloxychalcone. A significant glucose-lowering effect (P < 0.05) comparable with the standard glibenclamide has been observed for 4-ethyloxychalcone in an oral glucose tolerance test. 4-Ethyloxychalcone also produced a significant decrease (P < 0.05) in fasting blood glucose levels during the 42 days of treatment. Furthermore, a significant lowering (P < 0.05) of glycated hemoglobin (HbA1C ) level was also shown by 4-ethyloxychalcone after 42 days of treatment. Thus, 4-ethyloxychalcone might be regarded as a potential hypoglycemic agent that can act as a platform for the development of future antidiabetic drugs.

Similar content being viewed by others
References
L.J. Gray, J. Dales, E.M. Brady, K. Khunti, W. Hanif, M.J. Davies, Safety and effectiveness of non-insulin glucose-lowering agents in the treatment of people with type 2 diabetes who observe Ramadan: a systematic review and meta-analysis. Diabetes Obes. Metab. 17, 639–648 (2015)
M.G. Lepard, A.L. Joseph, A.A. Agne, A.L. Cherrington, Diabetes self-management interventions for adults with type 2 diabetes living in rural areas: a systematic literature review. Curr. Diabetes Rep. 15(6), 1–12 (2015)
J.W. Stevens, K. Khunti, R. Harvey, M. Johnson, L. Preston, H.B. Woods, M. Davies, E. Goyder, Preventing the progression to Type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res. Clin. Pract. 107, 320–331 (2015)
S.J. Chen, C. Aikawa, T. Matsui, Quantitative analysis of methylglyoxal, glyoxal and free advanced glycation end-products in the plasma of wistar rats during the oral glucose tolerance test. Biol. Pharm. Bull. 38, 336–339 (2015)
S. Menini, C. Iacobini, C. Ricci, C.B. Fantauzzi, G. Pugliese, Protection from diabetes-induced atherosclerosis and renal disease by d-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia 58, 845–853 (2015)
M. Brownlee, Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001)
A.M. Schmidt, S.D. Yan, S.F. Yan, D.M. Stern, The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta-Mol Cell Res. 1498, 99–111 (2000)
T. Takeuchi, O. Tsutsumi, Y. Ikezuki, Y. Takai, Y. Taketani, Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 51, 165–169 (2004)
K.M. Khan, M. Taha, F. Rahim, M.I. Fakhri, W. Jamil, M. Khan, S. Rasheed, A. Karim, S. Perveen, M.I. Choudhary, Acylhydrazide Schiff Bases: synthesis and antiglycation activity. J. Chem. Soc. Pak. 35, 929–937 (2013)
J.M. Gamble, A. Clarke, K.J. Myers, M.D. Agnew, K. Hatch, M.M. Snow, E.M. Davis, Incretin-based medications for type 2 diabetes: an overview of reviews. Diabetes Obes. Metab. 17, 649–658 (2015)
R.S. Rita, K. Dezaki, T. Kurashina, M. Kakei, T. Yada, Partial blockade of Kv2.1 channel potentiates GLP-1’s insulinotropic effects in islets and reduces its dose required for improving glucose tolerance in type 2 diabetic male mice. Endocrinology 156, 114–123 (2015)
W.M. Valenciaand, H. Florez, Pharmacological treatment of diabetes in older people. Diabetes Obes. Metab. 16, 1192–1203 (2014)
V. Eapen, D. Shiers, J. Curtis, Bridging the gap from evidence to policy and practice: reducing the progression to metabolic syndrome for children and adolescents on antipsychotic medication. Aust. N. Z. J. Psychiatry 47, 435–442 (2013)
K.L. Edward, B. Rasmussen, I. Munro, Nursing care of clients treated with atypical antipsychotics who have a risk of developing metabolic instability and/or type 2 diabetes. Arch. Psychiatr. Nurs. 24, 46–53 (2010)
T. Deng, H.J. Wang, C. Cai, Application of bis (oxazoline) in asymmetric beta-amination of chalcones. New J. Chem. 39, 102–105 (2015)
C. Karthikeyan, N.S.H.N. Moorthy, S. Ramasamy, U. Vanam, E. Manivannan, D. Karunagaran, P. Trivedi, Advances in chalcones with anticancer activities. Recent Patient Anti-Cancer Drug Discov. 10, 97–115 (2015)
G.A. Meshramand, V.A. Vala, Synthesis, characterization, and antimicrobial activity of benzimidazole-derived chalcones containing 1,3,4-oxadiazole moiety. Chem. Heterocycl. Compd. 51, 44–50 (2015)
B.I. Roman, T. De Ryck, S. Verhasselt, M.E. Bracke, C.V. Stevens, Further studies on anti-invasive chemotypes: an excursion from chalcones to curcuminoids. Bioorg. Med. Chem. Lett. 25, 1021–1025 (2015)
M.J. Matos, S. Vazquez-Rodriguez, E. Uriarte, L. Santana, Potential pharmacological uses of chalcones: a patent review (from June 2011–2014). Expert Opin. Ther. Patents 25, 351–366 (2015)
P. Singh, A. Anand, V. Kumar, Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 85, 758–777 (2014)
M. Ritter, R.M. Martins, D. Dias, C.M.P. Pereira, Recent advances on the synthesis of chalcones with antimicrobial activities: a brief review. Lett. Org. Chem. 11, 498–508 (2014)
Z. Nowakowska, A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 42, 125–137 (2007)
D.K. Mahapatra, V. Asati, S.K. Bharti, Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. Eur. J. Med. Chem. 92, 839–865 (2015)
S.M. Gomha, S.M. Riyadha, M.M. Abdalla, Solvent-drop grinding method: efficient synthesis, DPPH radical scavenging and anti-diabetic activities of chalcones, bis-chalcones, azolines, and bis-azolines. Curr. Org. Synth. 12, 220–228 (2015)
T. Enoki, H. Ofinogi, K. Nagamine, Y. Kudo, K. Sugiyama, M. Tanabe, E. Kobayashi, H. Sagawa, I. Kato, Antidiabetic activities of chalcones isolated from a Japanese herb, Angelica keiskei. J. Agric. Food Chem. 55, 6013–6017 (2007)
K. Kawanishi, H. Ueda, M. Moriyasu, Aldose reductase inhibitors from the nature. Curr. Med. Chem. 10, 1353–1374 (2003)
A. Abbas, S. Kalsoom, T.B. Hadda, M.M. Naseer, Evaluation of 4-alkoxychalcones as a new class of antiglycating agents: a combined experimental and docking study. Res. Chem. Intermed. 41, 6443–6462 (2015)
A. Hussain, M.K. Kashif, M.M. Naseer, U.A. Rana, S. Hameed, Synthesis and in vivo hypoglycemic activity of new imidazolidine-2,4-dione derivatives. Res. Chem. Intermed. 41, 7313–7326 (2015)
T.M. Babar, M.M. Naseer, F.I. Ali, N.H. Rama, T. Ben Hadda, Synthesis and effect of substituent position on anti-inflammatory activity of 3-(halobenzyl) isocarbostyrils. Med. Chem. Res. 23, 4607–4618 (2014)
T.M. Babar, M.M. Naseer, M.K. Rauf, H. Pervez, M. Ebihara, N.H. Rama, Synthesis of hexacyclic fused isocoumarin framework through selective domino multicyclizations under catalyst and solvent free conditions. Chin. Chem. Lett. 25, 1282–1286 (2014)
F. Anam, A. Abbas, K.M. Lo, Zia-ur-Rehman, S. Hameed, M.M. Naseer, Homologous 1,3,5-triarylpyrazolines: synthesis, CH···π interactions guided self-assembly and effect of alkyloxy chain length on DNA binding properties. New J. Chem. 38, 5617–5625 (2014)
A. Abbas, M.M. Naseer, Synthesis and anti-inflammatory activity of new N-acyl-2-pyrazolines bearing homologous alkyloxy side chains. Acta Chim. Slov. 61, 792–802 (2014)
K.L. Joyand, R. Kuttan, Anti-diabetic activity of Picrorrhiza kurroa extract. J. Ethnopharmacol. 67, 143–148 (1999)
J. Naowaboot, P. Pannangpetch, V. Kukongviriyapan, B. Kongyingyoes, U. Kukongviriyapan, Antihyperglycemic, antioxidant and antiglycation activities of mulberry leaf extract in streptozotocin-induced chronic diabetic rats. Plant Foods Hum. Nutr. 64, 116–121 (2009)
E.H. Alberton, R.G. Damazio, L.H. Cazarolli, L.D. Chiaradia, P.U. Leal, R.J. Nunes, R.A. Yunes, F.R.M.B. Silva, Influence of chalcone analogues on serum glucose levels in hyperglycemic rats. Chem. Biol. Interact. 171, 355–362 (2008)
R.G. Damazio, A.P. Zanatta, L.H. Cazarolli, L.D. Chiaradia, A. Mascarello, R.J. Nunes, R.A. Yunes, F.R.M.B. Silva, Antihyperglycemic activity of naphthylchalcones. Eur. J. Med. Chem. 45, 1332–1337 (2010)
M. Najafian, A. Ebrahim-Habibi, P. Yaghmaei, K. Parivar, B. Larijani, Core structure of flavonoids precursor as an antihyperglycemic and antihyperlipidemic agent: an in vivo study in rats. Acta Biochim. Pol. 57, 553–560 (2010)
A. Andrade-Cettoand, H. Wiedenfeld, Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J. Ethnopharmacol. 78, 145–149 (2001)
S. Lenzen, The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51, 216–226 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murtaza, B., Abbas, A., Aslam, A. et al. Evaluation of in vivo hypoglycemic potential of 4-ethyloxychalcone in alloxan-induced diabetic rats. Res Chem Intermed 42, 4161–4170 (2016). https://doi.org/10.1007/s11164-015-2266-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2266-0