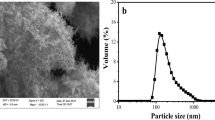
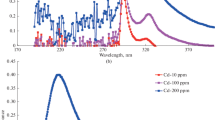
Abstract
Facilely synthesized zinc hydroxide nanoparticles by electro-dissolution of zinc sacrificial anodes were investigated for the adsorption of thorium (Th4+), uranium (U4+) and cerium (Ce4+) from aqueous solution. Various operating parameters such as effect of pH, current density, temperature, electrode configuration, and electrode spacing on the adsorption efficiency of Th4+, U4+ and Ce4+ were studied. The results showed that the maximum removal efficiency was achieved for Th4+, U4+ and Ce4+ with zinc as anode and stainless steel as cathode at a current density of 0.2 A/dm2 and pH of 7.0. First- and second-order rate equations were applied to study the adsorption kinetics. The adsorption process follows second order kinetics model with good correlation. The Langmuir, Freundlich adsorption models were applied to describe the equilibrium isotherms and the isotherm constants were determined. The experimental adsorption data were fitted to the Langmuir adsorption model. Thermodynamic parameters such as free energy (ΔG°), enthalpy (ΔH°), and entropy changes (ΔS°) for the adsorption of Th4+, U4+ and Ce4+ were computed to predict the nature of adsorption process. Temperature studies showed that the adsorption was endothermic and spontaneous in nature.












Similar content being viewed by others
References
D. Rana, T. Matsuura, M.A. Kassim, A.F. Ismail, Desalination 321, 77 (2013)
M. Saleem, M. Afzal, R. Qadeer, J. Hanif, J. Radloanal, Nuclear Chem. 172, 257 (1993)
S. Shankar Dubey, B. Sreenivasa Rao, J. Hazard Mater. 186, 1028 (2011)
Z. Xiaofei Zhang, W. Jun, L. Rumin, D. Qihui, L. Lianhe, N. J. Chem. 37, 3914 (2013)
M. Kalina, W.N. Wheelerb, G. Meinrath, J. Environ. Radioact. 78, 151 (2005)
M.A. Mohsen, F.H. Mohammed, J. Dispers. Sci. Technol. 34, 182 (2013)
H. Fatima, N. Djamel, A. Samira, B. Mahfoud, Desalin. Water Treat. 51, 583 (2013)
A. Kilincarslan Kaygun, S. Akyil, J. Hazard Mater. 147, 357 (2007)
N. Pan, J. Deng, D. Guan, Y. Jin, C. Xia, Appl. Surf. Sci. 287, 478 (2013)
D. Xu, C. Chen, X. Tan, J. Hu, X. Wang, Appl. Geochem. 22, 2892 (2007)
C.L. Chen, X.K. Wang, Appl. Geochem. 22, 436 (2007)
J. Li, Y. Zhang, Proced. Environ. Sci. 13, 1609 (2012)
L.W. Yu, S. Li-Juan, Z. Lu, G. Bo-Long, C. Su-Wen, W. Wang-Suo, Dalton Trans. 43, 3739 (2014)
E. Onder, A.S. Koparal, U.B. Ogutveren, Sep. Purif. Technol. 52, 527 (2007)
D.W. Miwa, G.R.P. Malpass, S.A.S. Machado, A.J. Motheo, Water Res. 40, 3281 (2006)
E.A. Vik, D.A. Carlson, A.S. Eikum, E.T. Gjessing, Water Res. 18, 1355 (1984)
S. Vasudevan, J. Lakshmi, J. Jayaraj, G. Sozhan, J. Hazard Mater. 164, 1480 (2009)
S. Vasudevan, S. Margrat Sheela, J. Lakshmi, G. Sozhan, J. Chem. Technol. Biotech. 85, 926 (2010)
J. Vanmuylder, M. Pourbaix, in Atlas of Electrochemical Equilibria in Aquous Solutions: Zinc, ed. by J. Vanmuylder, M. Pourbaix (Pergamon, New York, 1966)
P.K. Singh, B. Sushmita, A.L. Srivastava, Y.C. Sharma, RSC Adv. 5, 35365 (2015)
Y.S. Ho, G. McKay, Adsorpt. Sci. Technol. 16, 243 (1998)
H.M.F. Freundlich, J. Phys. Chem. A 57, 385 (1906)
P. Sathishkumar, M. Arulkumar, V. Ashokkumar, A. Rahim Mohd Yusoff, K. Murugesan, T. Palvannan, Z. Salam, F. Nasir Anic, T. Hadibarata, RSC Adv. 5, 30950 (2015)
M. Alkan, B. Kalay, M. Dogan, J. Hazard Mater. 153, 867 (2008)
A.K. Golder, A.N. Samantha, S. Ray, Sep. Purif. Technol. 52, 102 (2006)
M.J. Hernandez-Moreno, M.A. Ulibarri, J.L. Renon, C.J. Serna, Phys. Chem. Miner. 12, 34 (1985)
K.Y. Foo, B.H. Hameed, Chem. Eng. J. 87, 53 (2012)
Acknowledgments
The authors wish to express their gratitude to Dr. Vijayamohanan K. Pillai, Director, CSIR-Central Electrochemical Research Institute, Karaikudi to publish this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamaraj, R., Vasudevan, S. Facile one-pot synthesis of nano-zinc hydroxide by electro-dissolution of zinc as a sacrificial anode and the application for adsorption of Th4+, U4+, and Ce4+ from aqueous solution. Res Chem Intermed 42, 4077–4095 (2016). https://doi.org/10.1007/s11164-015-2259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2259-z