Abstract
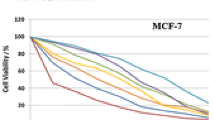
The mixed ligand Zn(II), Zr(IV), Pd(II), Ce(IV), Th(IV), and U(VI) complexes of gemifloxacin and N-donor 2,2′-bipyridine have been prepared. These complexes have been characterized by elemental, infrared (IR), ultraviolet–visible (UV–Vis), 1H nuclear magnetic resonance (NMR), mass spectroscopy, thermal, molar conductance and magnetic susceptibility analyses. The infrared spectra indicate that gemifloxacin acts as a bidentate deprotonated ligand bound to the metal through the pyridone oxygen and one carboxylate oxygen, whereas the 2,2′-bipyridine coordinates through the two nitrogen atoms. The central metal in each complex is six-coordinate and a slightly distorted octahedral geometry is proposed. The calculated bond length and force constant, F(U=O), in the uranyl complex are 1.751 Å and 641.04 N m−1, respectively. The thermodynamic parameters are calculated from the thermal analysis curves using the Coats–Redfern and Horowitz–Metzger methods. The antibacterial and antifungal activities of the ligand, metal salt and metal complexes have been tested. The data show that the complexes exhibit significant action against some of the four bacterial species but no effect on two species of fungi compared with gemifloxacin. The antitumor activity of the ligands and their complexes has been evaluated against breast (MCF-7) and colon (HCT-116) cancer cells.







Similar content being viewed by others
References
I. Turel, Coord. Chem. Rev. 232, 27–47 (2002)
V.T. Andriole, The quinolones: prospects, in The Quinolones, 3rd edn., ed. by V.T. Andriole (Academic, San Diego, 2000), pp. 477–495
J. Kuhlmann, H.G. Schaefer, D. Beermann, Clinical pharmacology, in Quinolone Antibacterials, Handbook of Experimental Pharmacology, vol. 127, ed. by J. Kuhlmann, A. Dalhoff, H.-J. Zeile (Springer, Berlin, 1998), pp. 339–406
D.M. Johnson, R.N. Jones, M.E. Erwin, Diagn. Microbiol. Infect. Dis. 33, 87–91 (1999)
R. Grossman, J. Rotschafer, J. Tan, Am. J. Med. 118, 29–38 (2005)
R.N. Patel, N. Singh, K.K. Shukla, V.L.N. Gundla, U.K. Chauhan, Spectrochim. Acta, Part A 63, 21–26 (2006)
B. Viossat, J. Daran, G. Savouret, G. Morgant, F.T. Greenaway, N. Dung, V.A. Pham-Tran, J.R.J. Sorenson, J. Inorg. Biochem. 96, 375–385 (2003)
F. Dimiza, A.N. Papadopoulos, V. Tangoulis, V. Psycharis, C.P. Raptopoulou, D.P. Kessissoglou, G. Psomas, Dalton Trans. 39, 4517–4528 (2010)
I. Turel, A. Golobic, A. Klavzar, B. Pihlar, P. Buglyo, E. Tolis, D. Rehder, K. Sepcic, J. Inorg. Biochem. 95, 199–207 (2003)
M.P. Lopez-Gresa, R. Ortiz, L. Perello, J. Latorre, M. Liu-Gonzalez, S. Garcia-Granda, M. Perez-Priede, E. Canton, J. Inorg. Biochem. 92, 65–74 (2002)
E.K. Efthimiadou, M.E. Katsarou, A. Karaliota, G. Psomas, J. Inorg. Biochem. 102, 910–920 (2008)
E.K. Efthimiadou, H. Thomadaki, Y. Sanakis, C.P. Raptopoulou, N. Katsaros, A. Scorilas, A. Karaliota, G. Psomas, J. Inorg. Biochem. 101, 64–73 (2007)
M.E. Katsarou, E.K. Efthimiadou, G. Psomas, A. Karaliota, D. Vourloumis, J. Med. Chem. 51, 470–478 (2008)
E.K. Efthimiadou, Y. Sanakis, M. Katsarou, C.P. Raptopoulou, A. Karaliota, N. Katsaros, G. Psomas, J. Inorg. Biochem. 100, 1378–1388 (2006)
G. Psomas, C.P. Raptopoulou, L. Iordanidis, C. Dendrinou-Samara, V. Tangoulis, D.P. Kessissoglou, Inorg. Chem. 39, 3042–3048 (2000)
C. Dendrinou-Samara, G. Psomas, C.P. Raptopoulou, D.P. Kessissoglou, J. Inorg. Biochem. 83, 7–16 (2001)
D.J. Beecher, A.C. Wong, Appl. Environ. Microbiol. 60, 1646–1651 (1994)
E. Fallik, J. Klein, S. Grinberg, E. Lomaniee, S. Lurie, A. Lalazar, Plant Dis. 77, 985–988 (1993)
T.A. Yousef, O.A. El-Gammal, S.F. Ahmed, G.M. Abu El-Reash, Spectrochim. Acta, Part A 135, 690–703 (2015)
Y. Saintigny, R.J. Monnat Jr, Sci. Aging Knowl. Environ. 13, 1–10 (2004)
T. Mosmann, J. Immunol. Methods 65, 55–63 (1983)
K. Saotome, H. Morita, M. Umeda, Toxicol. In Vitro 3, 317–321 (1989)
A.P. Wilson, in Cytotoxicity and Viability Assays in Animal Cell Culture: A Practical Approach, vol. 1, 3rd edn., ed. by J.R.W. Masters (Oxford University Press, Oxford, 2000)
M.S. Refat, Spectrochim. Acta, Part A 68, 1393–1405 (2007)
G.B. Deacon, R.J. Phillips, Coord. Chem. Rev. 33, 227–250 (1980)
S.A. Sadeek, W.H. EL-Shwiniy, J. Mol. Struct. 977, 243–253 (2010)
K. Nakamoto, P.J. McCarthy, S. FuJiwara, Y. Shimura, J. Fujita, C.R. Hare, Y. Saito, Spectroscopy and Structure of Metal Chelate Compounds (Wiley, New York, 1968)
R.M. Silverstein, G.C. Bassler, T.C. Morril, Spectroscopic Identification of Organic Compounds, 5th edn. (Wiley, New York, 1991)
W.M.I. Hassan, M.A. Badawy, G.G. Mohamed, H. Moustafa, S. Elramly, Spectrochim. Acta, Part A 111, 169–177 (2013)
W.H. Mahmoud, G.G. Mohamed, M.M.I. El-Dessouky, J. Mol. Struct. 1082, 12–22 (2015)
W.H. Mahmoud, G.G. Mohamed, M.M.I. El-Dessouky, Spectrochim. Acta, Part A 122, 598–608 (2014)
D. Zhong, Z. Chen, Y. Liu, X. Luo, C. Barta, H. Liang, J. Coord. Chem. 63, 3146–3154 (2010)
Y. Najajreh, J.M. Perez, C. Navarro-Ranninger, D. Gibson, J. Med. Chem. 45, 5189–5195 (2002)
P.A. Nguewa, M.A. Fuertes, S. Iborra, Y. Najajeh, D. Gibson, E. Matínez, C. Alonso, J.M. Pérez, J. Inorg. Biochem. 99, 727–736 (2005)
L.H. Jones, Spectrochim. Acta, Part A 15, 409–411 (1959)
S.P. Mcglynn, J.K. Smith, W.C. Neely, J. Chem. Phys. 35, 105–116 (1961)
F.D. Rochon, R. Melanson, J.P. Macouet, F. Belanger-Gariepy, A.L. Beauchamp, Inorg. Chim. Acta 108, 17–21 (1985)
T.G. Appleton, J.R. Hall, S.F. Ralph, Inorg. Chem. 24, 673–677 (1985)
M.A. Bennett, G.B. Robertson, A. Rokicki, W.A. Wickramasinghe, J. Am. Chem. Soc. 110, 7098–7105 (1988)
S.A. Sadeek, W.H. EL-Shwiniy, J. Mol. Struct. 981, 130–138 (2010)
S.A. Sadeek, W.H. EL-Shwiniy, M.S. El-Attar, Spectrochim. Acta, Part A 84, 99–110 (2011)
B. Macias, M.V. Villa, I. Rubio, A. Castineiras, J. Borras, J. Inorg. Biochem. 84, 163–170 (2001)
A.L. Ringer, C.D. Sherrill, R.A. King, T.D. Crawford, Int. J. Quantum Chem. 108, 1137–1140 (2008)
N.N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd edn. (Butterworth-Heinemann, London, 1997), pp. 320–321
M.S. Refat, I.M. El-Deen, I. Grabchev, Z.M. Anwer, S. El-Ghol, Spectrochim. Acta, Part A 72, 772–782 (2009)
S.A. Sadeek, W.H. EL-Shwiniy, W.A. Zordok, A.M. EL-Didamony, Spectrochim. Acta Part A 78, 854–867 (2011)
S. Kawahara, Nippon. Rinsho 56, 3096–3099 (1998)
B. Macias, M. Martinez, A. Sanchez, A. Dominguez-Gil, Int. J. Pharm. 106, 229–235 (1994)
S. Sagdinc, S. Bayarı, J. Mol. Struct. 691, 107–113 (2004)
A.W. Coats, J.P. Redfern, Nature 201, 68–69 (1964)
H.H. Horowitz, G. Metzger, Anal. Chem. 35, 1464–1468 (1963)
S.H. Guzar, Q.H. Jin, Chem. Res. Chin. Univ. 24, 143–147 (2008)
Z. Chohan, A. Munawar, C. Supuran, Met. Based Drugs 8, 137–143 (2001)
W. Hanna, M. Moawad, Trans. Met. Chem. 26, 644–651 (2001)
J. Iqbal, S. Tirmizi, F. Watto, M. Imran, M.H. Watto, S. Sharfuddin, S. Latif, Turk. J. Biol. 30, 1–4 (2006)
V. Singh, A. Katiyar, S. Singh, Bio Metals 21, 491–501 (2008)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadeek, S.A., Abd El-Hamid, S.M. & El-Shwiniy, W.H. Synthesis, spectroscopic characterization, thermal stability and biological studies of mixed ligand complexes of gemifloxacin drug and 2,2′-bipyridine with some transition metals. Res Chem Intermed 42, 3183–3208 (2016). https://doi.org/10.1007/s11164-015-2205-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2205-0