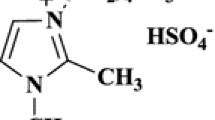
Abstract
In this study a synthetic method using TiCl3OTf-ionic liquid was reported for synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones (3,4-DHPMs). Condensation reactions were carried out for aldehydes, ethyl acetoacetate, and ammonium acetate. In the reaction 1-butyl-3-methylimidazolium chloride ([bmim]Cl) was used as an ionic liquid in the presence of TiCl3OTf catalyst under solvent-free conditions at 140 °C. The advantages of this method are high yield of product, short reaction time, and reusable catalyst. A mechanism was proposed for this condensation reaction, and all steps of the suggested mechanism were confirmed by density functional theory calculations using the B3LYP/6-311G level of theory.




Similar content being viewed by others
References
C.O. Kappe, Eur. Med. Chem. 35, 1043–1052 (2000)
C.O. Kappe, O.V. Shishkin, G. Uray, P. Verdino, Tetrahedron 56, 1859–1862 (2000)
P. Biginelli, Gazz. Chim. Ital. 23, 360–416 (1893)
M. Nasr-Esfahani, S.J. Hoseini, F. Mohammadi, Chin. J. Catal. 32, 1484–1489 (2011)
J.T. Starcevich, T.J. Laughlin, R.S. Mohan, Tetrahedron Lett. 54, 983–985 (2013)
R. Tayebee, M.M. Amini, M. Ghadamgahi, M. Armaghan, J. Mol. Catal. A Chem. 366, 266–274 (2013)
A. Farhadi, M.A. Takassi, L. Hejazi, Z. Naturforsch. 68b, 51–56 (2013)
H. Kiyani, M. Ghiasi, Res. Chem. Intermed. In press, doi:10.1007/s11164-014-1621-x (2014)
A.R. Katritzky, O. Meth-Cohn, C.W. Rees, Comprehensive Organic Functional Group Transformations (Pergamon, Oxford, 1995)
J. Noei, A.R. Khosropour, Tetrahedron Lett. 49, 6969–6971 (2008)
J. Noei, A.R. Khosropour, A. Mirjafari, Bull. Korean Chem. Soc. 33, 2102–2104 (2012)
R. Tayebee, M. Ghadamgahi, Arab. J. Chem. In press, doi:10.1016/j.arabjc.2012.12.001 (2013)
A. Debache, L. Chouguiat, R. Boulcina, B. Carbonib, Open Org. Chem. J. 6, 12–20 (2012)
G.H. Mahdavinia, H. Sepehrian, Chin. Chem. Lett. 19, 1435–1439 (2008)
X.L. Shi, H. Yang, M. Tao, W. Zhang, R. Soc. Chem. Adv. 3, 3939–3945 (2013)
K.K. Pasunooti, H. Chai, C.N. Jensen, B.K. Gorityala, S. Wang, X.-W. Liu, Tetrahedron Lett. 52, 80–84 (2011)
K.A. Dilmaghani, B. Zeynizadeh, H. Parsajam, Phosphorus Sulfur Silicon Relat. Elem. 187, 544–553 (2012)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, V.G. Zakrzewski, J.A. Montgomery Jr, R.E. Stratmann, J.C. Burant, S. Dapprich, J.M. Millam, A.D. Daniels, K.N. Kudin, M.C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G.A. Petersson, P.Y. Ayala, Q. Cui, K. Morokuma, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J. Ciolowski, J.V. Ortiz, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, J.L. Andres, M. Head-Gordon, E.S. Replogle, J.A. Pople, Gaussian 98 (Gaussian Inc., Pittsburg PA, 1998)
A. Farhadi, M.A. Takassi, P. Madmoli, J. Am. Sci. 8, 2024–2038 (2012)
Acknowledgments
The authors are grateful to the Islamic Azad University, Mahshahr branch, and the Petroleum University of Technology for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farhadi, A., Noei, J., Aliyari, R.H. et al. Experimental and theoretical study on a one-pot, three-component route to 3,4-dihydropyrimidin-2(1H)-ones/thiones TiCl3OTf-[bmim]Cl. Res Chem Intermed 42, 1401–1409 (2016). https://doi.org/10.1007/s11164-015-2092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2092-4