Abstract
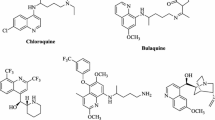
In this work, a simple, rapid and efficient method for the preparation of benzimidazoles and quinoxalines from the condensation of o-phenylene diamines with aldehydes and/or 1,2-dicarbonyl compounds in the presence of sulfonated rice husk ash (RHA-SO3H) as an efficient green catalyst is reported. RHA-SO3H can be easily prepared using a readily available organic compound by simple modification of rice husk ash. All reactions are performed under mild reaction conditions with high to excellent yields. The method is applicable to aromatic, unsaturated and hetero aromatic aldehydes. The advantages of this method are short reaction times, milder conditions, easy work-up, solvent-free conditions and catalyst reusability.



Similar content being viewed by others
References
B. Narasimhan, D. Sharma, P. Kumar, Med. Chem. Res. 21, 269 (2012)
M. Gaba, S. Singh, C. Mohan, Eur. J. Med. Chem. 76, 494 (2014)
K.P. Barot, S. Nikolova, I. Ivanov, M.D. Ghate, Mini-Rev. Med. Chem. 13, 1421 (2013)
F. Fei, Z.M. Zhou, Expert Opin. Ther. Pat. 23, 1157 (2013)
A.A. Ivanov, O.Y. Susova, V.I. Salyanov, K.I. Kirsanov, A.L. Zhuze, J. Biomol. Struct. Dyn. 31, 52 (2013)
J. Jayabharathi, V. Thanikachalam, K. Jayamoorthy, R. Sathishkumar, Spectrochim. Acta A 97, 384 (2012)
E.J. Hanan, B.K. Chan, A.A. Estrada, D.G. Shore, J.P. Lyssikatos, Synlett 18, 2759 (2010)
D. Yang, D. Fokas, J. Li, L. Yu, C.M. Baldino, Synthesis 1, 47 (2005)
W. Cui, R.B. Kargbo, Z. Sajjadi-Hashemi, F. Ahmed, J.F. Gauuan, Synlett 23, 247 (2012)
J. Sluiter, J. Christoffers, Synlett 1, 63 (2009)
B.C. Wray, J.P. Stambuli, Org. Lett. 12, 4576 (2010)
P. Saha, T. Ramana, N. Purkait, M.A. Ali, R. Paul, T. Punniyamurthy, J. Org. Chem. 74, 8719 (2009)
G. Bastug, C. Eviolitte, I.E. Markó, Org. Lett. 14, 3502 (2012)
K. Bahrami, M.M. Khodaei, I. Kavianinia, Synthesis 417, 417 (2007)
A. Go, G. Lee, J. Kim, S. Bae, B.M. Lee, B.H. Kim, Tetrahedron 71, 1215 (2015)
F.A.R. Rodrigues, I.D.S. Bomfima, B.C. Cavalcanti, C.D. Pessoa, J.L. Wardell, S.M.S.V. Wardell, A.C. Pinheiro, C.R. Kaiser, Bioorg. Med. Chem. Lett. 24, 934 (2014)
J. Brown Desmond, C.T. E., A. Ellman Jonathan, The Chemistry of Heterocyclic Compounds (Wiley, New York) (2004)
D. Aparicio, O.A. Attanasi, P. Filippone, R. Ignacio, S. Lillini, F. Mantellini, F. Palacios, J.M. de los Santos, J. Org. Chem. 71, 5897 (2006)
S. Antoniotti, E. Dunach, Tetrahedron Lett. 43, 3971 (2002)
Y.V.D. Nageswar, K.H.V. Reddy, K. Ramesh, S.N. Murthy, ChemInform 44 (2013)
M. Lande, M. Navgire, S. Rathod, J. Ind. Eng. Chem. 18, 277 (2012)
A. Teimouri, A. Najafi Chermahini, H. Salavati, L. Ghorbanian, J. Mol. Catal. A Chem. 373, 38 (2013)
A. Hasaninejad, A. Zare, M. Shekouhy, A.R. Moosavi-Zare, Eur.-J. Chem. 6(S1), S247 (2009)
M.M. Heravi, Kh. Bakhtiari, M.H. Tehrani, N.M. Javadi, H.A. Oskooie, ARKIVOC 16, 247 (2006)
A. Zare, A. Hasaninejad, A. Parhami, A.R. Moosavi-Zare, J. Serb. Chem. Soc. 75, 1315 (2010)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, J. Mol. Catal. A Chem. 363, 10 (2012)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, A.R. Aliakbar, C. R. Chim. 16, 207 (2013)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, C. R. Chim. 16, 945 (2013)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, Chin. J. Catal. 34, 2200 (2013)
F. Shirini, M. Mamaghani, M. Seddighi, Catal. Commun. 36, 31 (2013)
M. Seddighi, F. Shirini, M. Mamaghani, RSC Adv. 3, 24046 (2013)
R.R. Nagawade, D.B. Shinde, Indian J. Chem. 46B, 349 (2007)
S.B. Rathod, M.K. Lande, B.R. Arbad, Bull Korean Chem. Soc. 31, 2835 (2010)
H. Eshghi, M. Rahimizadeh, A. Shiri, P. Sedaghat, Bull Korean Chem. Soc. 33, 515 (2012)
L.S. Gadekar, B.R. Arbad, M.K. Lande, Chin. Chem. Lett. 21, 1053 (2010)
S.E. López, J. Restrepo, B. Pérez, S. Ortiz, J. Salazar, Bull Korean Chem. Soc. 30, 1628 (2009)
P. Gogoi, D. Konwar, Tetrahedron Lett. 47, 79 (2006)
Y. Venkateswarlu, S. Ramesh Kumar, P. Leelavathi, Org. Med. Chem. Lett. 1, 11 (2013)
K. Bahrami, M.M. Khodaei, A. Nejati, Green Chem. 12, 1237 (2010)
J. Yuan, Z. Zhao, W. Zhu, H. Li, X. Qiao, Y. Xu, Tetrahedron 69, 7026 (2013)
P. Karastatiris, J.A. Mikroyannidis, I.K. Spiliopoulos, Macromolecules 37, 7867 (2004)
M.A. Chari, D. Shobha, T. Sasaki, Tetrahedron Lett. 52, 5575 (2011)
B. Das, H. Holla, Y. Srinivas, Tetrahedron Lett. 48, 61 (2007)
M.A. Chari, Z.A. Mosaa, D. Shobha, S. Malayalam, Int. J. Org. Chem. 3, 243 (2013)
R.S. Joshi, P.G. Mandhane, S.K. Dabhade, C.H. Gill, J. Chin. Chem. Soc. 57, 1227 (2010)
Acknowledgments
We are thankful to the University of Guilan Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shamsi-Sani, M., Shirini, F., Abedini, M. et al. Synthesis of benzimidazole and quinoxaline derivatives using reusable sulfonated rice husk ash (RHA-SO3H) as a green and efficient solid acid catalyst. Res Chem Intermed 42, 1091–1099 (2016). https://doi.org/10.1007/s11164-015-2075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2075-5