Abstract
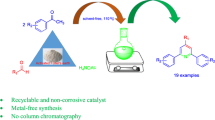
Carbon-based solid acid has been used as a mild and efficient heterogeneous catalyst for the synthesis of tetrahydrobenzo[b]pyran derivatives via a one-vessel, three-component cyclocondensation of dimedone, aryl aldehydes, and malononitrile with high atom economy ranging from 94.2 to 95.4 %. The remarkable features of this new method are short reaction times, high yields, clean reaction profiles, and simple work-up procedure. The reactions were performed in ethanol and the catalyst could be recycled after a simple work-up, and used for many runs without significant loss of its activity. However, using aliphatic aldehydes, moderate yields of the products were obtained. The structure of the catalyst was confirmed by FT-IR spectroscopy, energy dispersive X-ray, X-ray diffraction, and the N2 adsorption/desorption analysis techniques.







Similar content being viewed by others
References
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
G.R. Green, J.M. Evans, A.K. Vong, in Comprehensive Heterocyclic Chemistry II, ed. by A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Pergamon, Oxford, 1995), pp. 469–500
K. Rehse, W. Schinkel, Arch. Pharm. 316, 988 (1983)
I. Fatima, R. Saxena, G. Kharkwal, M.K. Hussain, N. Yadav, K. Hajela, P.L. Sankhwar, A. Dwivedi, J. Steroid Biochem. Mol. Biol. 138, 123 (2013)
A.E.F.G. Hammam, O.I. Abd El-Salam, A.M. Mohamed, N.A. Hafez, Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 44, 1887 (2005)
D. Armetso, W.M. Horspool, N. Martin, A. Ramos, C. Seoane, J. Org. Chem. 54, 5190 (1989)
S. Hatakeyama, N. Ochi, H. Numata, S. Takano, J. Chem. Soc. Chem. Commun. 1202 (1988)
C.S. Konkoy, D.B. Fick, S.X. Cai, N.C. Lan, J.F.W. Keana, PCT Int. Appl. WO Patent 200075123. Chem. Abstr. 134, 29313a (2001)
S. Paul, S. Ghosh, P. Bhattacharyya, A.R. Das, RSC Adv. 3, 14254 (2013)
M. Amirnejad, M.R. Naimi-Jamal, H. Tourani, H. Ghafuri, Monatsh. Chem. 144, 1219 (2013)
R.S. Bhosale, C.V. Magar, K.S. Solanke, S.B. Mane, S.S. Choudhary, R.P. Pawar, Synth. Commun. 37, 4353 (2007)
J.T. Li, W.Z. Xu, L.C. Yang, T.S. Li, Synth. Commun. 34, 4565 (2004)
J. Zheng, Y. Li, Mendeleev Commun. 21, 280 (2011)
S. Rathod, B. Arbad, M. Lande, Chin. J. Catal. 31, 631 (2010)
M. Hosseini-Sarvari, S. Shafiee-Haghighi, Chem. Heterocycl. Compd. 48, 1307 (2012)
P. Bhattacharyya, K. Pradhan, S. Paul, A.R. Das, Tetrahedron Lett. 53, 4687 (2012)
S. Nemouchi, R. Boulcina, B. Carboni, A. Debache, C. R. Chim. 15, 394 (2012)
S. Gurumurthi, V. Sundari, R. Valliappan, E. J. Chem. 6, S466 (2009)
R. Hekmatshoar, S. Majedi, Kh Bakhtiari, Catal. Commun. 9, 307 (2008)
D. Tahmassebi, J.A. Bryson, S.I. Binz, Synth. Commun. 41, 2701 (2011)
Y. Li, B. Du, X. Wang, D. Shi, S. Tu, J. Heterocycl. Chem. 43, 685 (2006)
S. Khaksar, A. Rouhollahpour, S. Mohammadzadeh Talesh, J. Fluorine Chem. 141, 11 (2012)
V.S. Gerard, F. Notheisz, Heterogeneous Catalysis in Organic Chemistry (Elsevier, San Diego, 2000)
K. Wilson, J.H. Clark, Pure Appl. Chem. 72, 1313 (2000)
N. Seifi, M.H. Zahedi-Niaki, M.R. Barzegari, A. Davoodnia, R. Zhiani, A.A. Kaju, J. Mol. Catal. A Chem. 260, 77 (2006)
M. Zeinali-Dastmalbaf, A. Davoodnia, M.M. Heravi, N. Tavakoli-Hoseini, A. Khojastehnezhad, H.A. Zamani, Bull. Korean Chem. Soc. 32, 656 (2011)
A. Davoodnia, M. Bakavoli, R. Moloudi, M. Khashi, N. Tavakoli-Hoseini, Chin. Chem. Lett. 21, 1 (2010)
N. Tavakoli-Hoseini, A. Davoodnia, Asian J. Chem. 22, 7197 (2010)
A. Davoodnia, A. Khojastehnezhad, N. Tavakoli-Hoseini, Bull. Korean Chem. Soc. 32, 2243 (2011)
A. Davoodnia, Synth. React. Inorg. Met-Org. Nano-Met. Chem. 42, 1022 (2012)
N. Mohammadzadeh-Dehsorkh, A. Davoodnia, N. Tavakoli-Hoseini, M. Moghaddas, Synth. React. Inorg. Met-Org. Nano-Met. Chem. 41, 1135 (2011)
A. Davoodnia, A. Zare-Bidaki, H. Behmadi, Chin. J. Catal. 33, 1797 (2012)
M. Hara, T. Yoshida, A. Takagaki, T. Takata, J.N. Kondo, S. Hayashi, K. Domen, Angew. Chem. Int. Ed. 43, 2955 (2004)
A. Davoodnia, A. Tavakoli-Nishaburi, N. Tavakoli-Hoseini, Bull. Korean Chem. Soc. 32, 635 (2011)
M. Moghaddas, A. Davoodnia, M.M. Heravi, N. Tavakoli-Hoseini, Chin. J. Catal. 33, 706 (2012)
N. Tavakoli-Hoseini, A. Davoodnia, Chin. J. Chem. 29, 203 (2011)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985)
Acknowledgment
The authors express their gratitude to the Islamic Azad University, Mashhad Branch for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghaddas, M., Davoodnia, A. Atom-economy click synthesis of tetrahydrobenzo[b]pyrans using carbon-based solid acid as a novel, highly efficient and reusable heterogeneous catalyst. Res Chem Intermed 41, 4373–4386 (2015). https://doi.org/10.1007/s11164-014-1536-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1536-6