Abstract
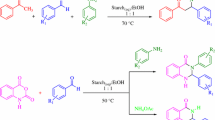
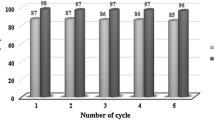
Starch sulfate as bio-supported solid acid catalyst was applied to synthesis of heterocyclic compounds such as 2,3-dihydroquinazolin-4(1H)-one, 2-substituted-1,2,3,4-tetrahydro-4-quinazolinones, 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one, 1,8-dioxo-octahydroxanthenes, and 5H-dibenzo[b,i]xanthene-5,7,12,14(13H)-tetraone derivatives under thermal solvent-free conditions. In all these synthetic methods, the work-up is simple and the catalyst can be easily separated from the product and reused for several times without any loss of its activity. The present protocol with starch sulfate as an effective catalyst is convincingly superior to the recently reported catalytic methods with respect to reaction times and yields of the obtained products.







Similar content being viewed by others
References
W. Zhang, W. Cue Berkeley, Green Techniques for Organic Synthesis and Medicinal Chemistry (Wiley, Chichester, 2012)
P. Zhou, H. Wang, J. Yang, J. Tang, D. Sun, W. Tang, Ind. Eng. Chem. Res. 5743, 51 (2012)
A. Buleon, P. Colonna, V. Planchot, S. Ball, Int. J. Biol. Macromol. 23, 85–112 (1998)
R.L. Whistler, E.F. Paschall, Starch: Chemistry and Technology (Academic Press, New York, 1965)
A.K. halafi-Nezhad, F. Panahi, J Org. Met. Chem. 141, 717 (2012)
S. Kumar, S.L. Jain, B. Sain, Catal. Today 204, 198 (2012)
A. Shaabani, A. Rahmati, Z. Badri, Catal. Commun. 13, 9 (2008)
H. Wu, X.M. Chen, Y. Wan, H.Q. Xin, Q. Lian, L. YE, Asian J. Chem. 2815, 21 (2009)
A. Kumar, S. Srivastava, S. Garima, Tetrahedron Lett. 517, 51 (2010)
Y. Wana, W. Lina, X.M. Chena, Y. Shena, H.Q. Xina, H.H. Xua, R. Yuana, L.L. Panga, R. Maa, C.H. Yuea, W. Yina, R.C. Boa, H. Wu, Lett. Org. Chem. 456, 6 (2009)
H.R. Shaterian, M. Arman, F. Rigi, J. Mol. Liq. 145, 158 (2011)
H.R. Shaterian, F. Rigi, Starch 340, 63 (2011)
H.R. Shaterian, F. Rigi, M. Arman, Chem Sci Trans. 155, 1 (2012)
H.R. Shaterian, F. Rigi, Chin. J. Chem. 695, 30 (2012)
J.X. Chen, D. Wu, F. He, M.C. Liu, H. Wu, J.C. Ding, W.K. Su, Tetrahedron Lett. 3814, 49 (2008)
L.-M. Wang, HuL Shao, J.-H. Yu, T. Zhang, J. Fluorine Chem. 1139, 129 (2008)
M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgarya, A.A. Mohammadi, Tetrahedron Lett. 6123, 46 (2005)
M. Dabiri, P. Salehi, M. Baghbanzadeh, M.A. Zolfigol, M. Agheb, S. Heydari, Catal. Commun. 785, 9 (2008)
A. Shaabani, A. Rahmati, R. Moghimi, JCR. Chimie. 759, 11 (2008)
P. Salehi, M. Dabiri, M. Baghbanzadeh, M. Bahramnejad, Synth. Commun. 2287, 36 (2006)
P. Salehi, M. Dabiri, M. A. Zolfigol, M. Baghbanzadeh, Synlett. 1155–1157 (2005)
M.P. Surpur, P.R. Single, S.B. Patil, S.D. Samat, Synth. Commun. 1965, 37 (2007)
M. Baghbanzadeh, P. Salehi, M. Dabiri, G. Kozehgarya, Synthesis 344, 2 (2006)
H.R. Shaterian, A.R. Oveisi, M. Honarmand, Synth. Commun. 1231, 40 (2010)
J.X. Chen, H.Y. Wu, W.K. Su, Chin. Chem. Lett. 18, 536 (2007)
J.X. Chen, D. Wu, F. He, M.C. Liu, H. Wu, J.C. Ding, W.K. Su, Tetrahedron Lett. 3814, 49 (2008)
M.P. Surpur, P.R. Single, S.B. Patil, S.D. Samat, Synth. Commun. 1965, 37 (2007)
F. Li, Y. Feng, Q. Meng, W. Li, Z. Li, Q. Wang, F. Tao, Arkivoc i, 40–46 (2007)
A. Shaabani, A. Maleki, H. Mofakham, Synth. Commun. 3751, 38 (2008)
G.C. Nandi, S. Samai, R. Kumar, M.S. Singh, Tetrahedron 7129, 65 (2009)
A. Sethukumar, M. Millet, B. Chandy, A. Prakasam, R. Pallepogu, Struct. Chem. 671, 22 (2011)
Z.H. Zhang, P. Zhang, S.H. Yang, H.J. Wang, J. Deng, J. Chem. Sci. 427, 122 (2010)
Z. Noroozi Tisseh, A. Bazgir, Dyes Pigm. 258, 83 (2009)
H.R. Tavakoli, H. Zamani, M.H. Ghorbani, H. Etedali Habibabadi, Iran. J. Org. Chem. 2118, 2 (2009)
H.A. Oskooie, L. Tahershamsi, M.M. Heravi, B. Baghernejad, Eur. J. Chem. 717, 7 (2010)
Acknowledgment
We are grateful to the University of Sistan and Baluchestan Research Council for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaterian, H.R., Rigi, F. An efficient synthesis of quinazoline and xanthene derivatives using starch sulfate as a biodegradable solid acid catalyst. Res Chem Intermed 41, 721–738 (2015). https://doi.org/10.1007/s11164-013-1223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1223-z