Abstract
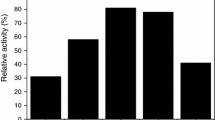
Fe2O3/MgO systems were prepared by the incipient impregnation, coprecipitation, and hydrothermal techniques. The solids obtained were calcined at 500–900 °C then characterized by use of XRD and EDX, by measurement of specific surface area (S BET), and by use as catalysts for H2O2 decomposition. The catalysts contained the nanosized MgO phase in addition to the MgFe2O4 phase. The concentration of surface iron species was highest for catalysts prepared by the coprecipitation method; these catalysts also had the highest specific surface area. The lattice constant of the MgO lattice in the Fe2O3/MgO system depends on both the method of preparation and calcination temperature. Catalysts prepared by the hydrothermal method were most active in H2O2 decomposition. The apparent activation energy and the mechanism of this reaction were not altered by variation of the method of preparation.




Similar content being viewed by others
References
V.R. Choudhary, M.Y. Pandit, Appl. Catal. 71, 265 (1991)
W.C. Choi, J.S. Kim, T.H. Lee, S.I. Woo, Catal. Today 63, 229 (2000)
Th. El-Nabarawy, A.M. Youssef, S.A.S. Ahmed, Adsorp. Sci. Technol. 19, 159 (2001)
E. Ruckenstein, H.Y. Wang, Appl. Catal. A 198, 33 (2000)
S.A. El-Molla, Appl. Catal. A 280, 189 (2005)
A. Khaleel, I. Shehadil, M. Al-Shamisi, Colloid. Surf. A 355, 75 (2010)
J.-L. Cao, Y. Wang, X.-L. Yu, S.-R. Wang, S.-H. Wu, Z.-Y. Yuan, Appl. Catal. B 79, 26 (2008)
S. Al-Sayari, A.F. Carley, S.H. Taylor, G.J. Hutchings, Top. Catal. 44, 123 (2007)
H.C. Wang, S.H. Chang, P.C. Hung, J.F. Hwang, M.B. Chang, Chemosphere 71, 388 (2008)
J.W. Geus, Appl. Catal. 15, 313 (1986)
O.K. Tan, W. Zhu, Q. Yan, L.B. Kong, Sens. Actuators B 65, 361 (2000)
J.S. Jiang, X.L. Yang, L. Gao, J.K. Guo, Mater. Sci. Eng. A 392, 179 (2005)
G.A. El-Shobaky, N.R.E. Radwan, F.M. Radwan, Thermochim. Acta 380, 27 (2001)
N. Koga, T. Tsutaoka, J. Magn. Magn. Mater. 313, 168 (2007)
B.Q. Xu, J.M. Wei, H.Y. Wang, K.Q. Sun, Q.M. Zhu, Catal. Today 68, 217 (2001)
V.V. Chesnokov, A.F. Bedilo, D.S. Heroux, I.V. Mishakov, K.J. Klabunde, J. Catal. 218, 438 (2003)
I.V. Mishakov, A.F. Bedilo, R.M. Richards, V.V. Chesnokov, A.M. Volodin, V.I. Zaikovskii, R.A. Buyanov, K.J. Klabunde, J. Catal. 206, 40 (2002)
W.F. Smith, Principle of Materials Science Engineering (McGraw–Hill Book Company, Singapore, 1986)
O. Koper, Ph.D. thesis, Department of Chemistry, Kansas State University, Manhattan, Kansas, USA, 1996
J. Stark, M.S. thesis, Department of Chemistry, Kansas State University, Manhattan, Kansas, USA, 1995
A. Khakel, W. Li, K. Klabunde, Nanostructured Mater. 12, 463 (1999)
D. Zhang, K. Klabunde, C. Sorensen, Nanostructured Mater. 12, 1053 (1999)
J.G. Seo, M.H. Youn, S. Park, J.C. Jung, P. Kim, I.K. Song, J. Power Sources 186, 178 (2009)
G. Li, L. Hu, J.M. Hill, Appl. Catal. A 301, 16 (2006)
Y. Ding, G. Zhang, H. Wu, B. Hai, L. Wang, Y. Qian, Chem. Mater. 13, 435 (2001)
M. El-Shall, W. Slack, W. Vann, D. Kane, D. Hanley, J. Phys. Chem. 98, 3067 (1998)
J.G. Seo, M.H. Youn, H.-I. Lee, J.J. Kim, E. Yang, J.S. Chung, Chem. Eng. J. 141, 298 (2008)
L. Diamandescu, M. Feder, D.T. Mihaila, F. Vasiliu, Appl. Catal. A 325, 270 (2007)
J.H. Lin, S.F. Liu, Q.M. Cheng, X.L. Qian, L.Q. Yang, M.Z. Su, J. Alloys Compd. 249, 237 (1997)
S.J. Shin, Y.H. Kim, C.W. Kim, H.G. Cha, Y.J. Kim, Y.S. Kang, Current Appl. Phys. 7, 404 (2007)
N. Teshimal, Z. Genfa, P.K. Dasgupta, Anal. Chim. Acta 510, 9 (2004)
K. Byrappa, T. Adschiri, Prog. Cryst. Growth Charact. Mater. 53, 117 (2007)
A.E. Sanli, A. Aytac, Int. J. Hydrogen Energy 36, 869 (2011)
M. Kitis, S.S. Kaplan, Chemosphere 68, 1846 (2007)
S.P. Jiang, W.R. Ashton, A.C.C. Tseung, J. Catal. 131, 88 (1991)
B.D. Cullity, Elements of X-Ray Diffraction, 2nd edn. (Addison-Wesley, Reading, 1978)
R.C. Weast, Handbook of Chemistry and Physics, 65th edn. (CRC, Florida, 1984–1985), p. F-165
S. Brunauer, P.H. Emmet, E. Teller, J. Am. Chem. Soc. 60, 309 (1938)
P. Decyk, M. Trejda, M. Ziolek, J. Kujawa, K. Głaszczka, M. Bettahar, S. Monteverdi, M. Mercy, J. Catal. 219, 146 (2003)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Molla, S.A., Fagal, G.A., Hassan, N.A. et al. Effect of the method of preparation on the physicochemical and catalytic properties of nanosized Fe2O3/MgO. Res Chem Intermed 41, 679–689 (2015). https://doi.org/10.1007/s11164-013-1220-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1220-2