Abstract
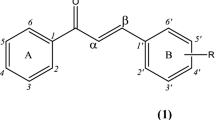
A series of novel 3-[N, N-bis(2-hydroxyethyl)-amino]-chalcone derivatives 3a–3j were synthesized by the aldol condensation of [N, N-bis(2-hydroethyl)-3-amino]-acetophenone 2 with aromatic aldehydes. Their structures were further confirmed by ESI-HRMS, 1H NMR, IR and elemental analysis. X-ray analysis reveals crystal 3b is a monoclinic system with P21/n space group. The antimicrobial activities of the newly synthesized chalcones in vitro were evaluated and the results indicated that most compounds presented moderate to good antimicrobial activities, especially the antifungal capability. Compounds 3a, 3d, 3f and 3g revealed obvious potency against Candida albicans with MIC values of 32 μg/mL, which were better compared with others.



Similar content being viewed by others
References
P.B. Bandgar, S.A. Patil, R.N. Gacche, B.L. Korbad, S.B. Hote, S.N. Kinkar, S.S. Jalde, Bioorg. Med. Chem. Lett. 20, 730 (2010)
L.M. Ni, C.Q. Meng, J.A. Sikorski, Expert Opin. Ther. Pat. 14, 1669 (2004)
X.F. Zhu, B.F. Xie, J.M. Zhou, G.K. Feng, Z.C. Liu, X.Y. Wei, F.X. Zhang, M.F. Liu, Y.X. Zeng, Mol. Pharmacol. 67, 1444 (2005)
S.F. Nielsen, S.B. Christensen, G. Cruciani, A. Kharazmi, T. Liljefors, J. Med. Chem. 41, 4819 (1998)
J.R. Dimmock, N.M. Kandepu, M. Hetherington, J.W. Quail, U. Pugazhenthi, A.M. Sudom, M. Rose, P. Chamankhah, E. Pass, T.M. Allen, S. Halleran, J. Szydlowski, B. Mutus, M. Tannous, E.K. Manavathu, T.G. Myers, E.D. Clercq, J. Balzarini, J. Med. Chem. 41, 1014 (1998)
O. Sabzevari, G. Galati, M.Y. Moridani, A. Siraki, P.J. O’Brien, Chem. Biol. Interact. 148, 57 (2004)
Y.M. Lin, Y. Zhou, M.T. Flavin, L.M. Zhou, W. Niea, F.C. Chen, Bioorg. Med. Chem. 10, 2795 (2002)
M. Liu, P. Wilairat, M.L. Go, J. Med. Chem. 44, 4443 (2001)
B. Madan, S. Batra, B. Ghosh, Mol. Pharmacol. 58, 526 (2000)
P.M. Sivakumar, G. Iyer, L. Natesan, M. Doble, Appl. Sur. Sci. 256, 6018 (2010)
P.M. Sivakumar, S. Priya, M. Doble, Chem. Biol. Drug Des. 73, 403 (2009)
J.R. Wingard, H.L. Leather, Oncol. 15, 351 (2001)
A.R. Gomes, H. Westh, H. Lencastre, Antimicrob. Agents Chemother. 50, 3237 (2006)
K. Coleman, Drug Discov. Today Ther. Strateg. 1, 455 (2004)
E.A. Palombo, S.J. Semple, J. Ethnopharmacol. 77, 151 (2001)
M.X. Yang, X. Lin, P. Yu, L.J. Chen, S.X. Liu, Chin. J. Chem 23, 1407 (2005)
NCCLS, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically M7-A5, vol. 20 (National Committee on Clinical Laboratory Standards, Wayne, 2000), p. 2
Y.K. Qi, B. Jiao, X.D. Ma, W.P. Cui, S.T. Ma, Arch. Pharm. Chem. Life Sci. 8, 458 (2010)
S. Wattanasin, W.S. Murphy, Synthesis 8, 647 (1980)
B.D. Palmer, W.R. Wilson, S.M. Pullen, W.A. Denny, J. Med. Chem. 33, 112 (1990)
G.H. Wu, L. Wang, S.M. Wang, L. Li, X.Y. Xu, J.X. Yang, Chin. J. Syn. Chem. 17, 503 (2009). (in Chinese)
E.D. D’silva, G.K. Podagatlapalli, S.V. Rao, D. Narayana, S.M. Dharmaprakash, Cryst. Growth Des. 11, 5362 (2011)
B.K. Sarojini, B. Narayana, B.V. Ashalatha, J. Indira, K.G. Lobo, J. Cryst. Growth 295, 54 (2006)
W.C. Huang, C.H. Lee, J.W. Liu, J. Microbiol. Immunol. Infect. 43, 470 (2010)
P.L. Zhao, C.L. Liu, W Huang, Y.Z. Wang, G.F. Yang, J. Agric. Food Chem. 55, 5697 (2007)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21172178). The authors are grateful to Prof. KeWu Yang for his useful comments on biological activity test.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, X., Yang, B., Cheng, Z. et al. Synthesis and antimicrobial activity of novel chalcone derivatives. Res Chem Intermed 40, 1715–1725 (2014). https://doi.org/10.1007/s11164-013-1076-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1076-5