Abstract
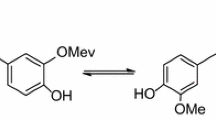
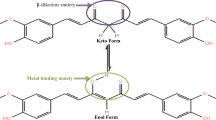
The antioxidant activity of three extracted curcuminods, namely, curcumin [(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5dione] (C), demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC), were studied with the DPPH, hydrogen peroxide and nitric oxide radical methods, and compared with the known antioxidant ascorbic acid. Structures for the extracted curcuminods are proposed on the basis of spectroscopic evidence. Curcumin molecule stability was studied in the gaseous state, and it has two isomers [the diketone form, curcumin(I), and the enol form, curcumin(II)] through the theoretical study by relying on the results of density functions theory (DFT). The results of each of the total energy and the high occupied molecular orbital (HOMO) to curcumin(I) are more stable than to curcumin(II). The increase of the amount of total energy is −0.01301141 a.u., or equivalent, −8,164.783 cal mol−1. The HOMO level is −0.35865 eV, also the thermodynamic values (the change in entropy ∆S and the change in enthalpy ∆H) of the isomerization conversion form curcumin(II) to curcumin(I) spontaneous and endothermic reaction, of ∆S and ∆H are −3.136 cal mol−1 K−1 and −0.673 kcal mol−1, respectively. The results showed that the wavelength for greatest absorption (λ max) of the enol form curcumin(II) is longer than curcumin(I). This is due to the formation of a new double bond which leads to the association distribution of the electronic density along the molecule in the enol form.








Similar content being viewed by others
References
P. Scartezzini, E. Speroni, Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 71, 23–43 (2000)
H.P.T. Ammon, M.A. Wahl, Pharmacology of Curcuma longa. Planta Med. 57(1991), 1–7 (1991)
K. Yoshinobu, S. Yuriko, W. Noriko, O. Yoshiteru, H. Hiroshi, Planta Med. 49, 185–187 (1983)
R. Kuttan, P. Bhanumathy, K. Nirmala, M. George, Cancer Lett. 29, 197–202 (1985)
R. Selvam, L. Subramanian, R. Gayathri, N. Angayarkanni, J. Ethnopharmacol. 47, 59–67 (1995)
F. Bonte, M.S. Noel-Hudson, J. Wepierre, A. Meybeck, Planta Med. 63, 265–266 (1997)
M. Kolev, Int. J. Quantum Chem. 102(6), 1069–1079 (2005)
J. Miłobȩdzka, St. Kostanecki, V. Lampe, Ber Dtsch Chem Ges 43(2), 2163–2170 (1910)
A. Kadhum, A. Mohamad, A. Al-Amiery, Molecules 16, 6969–6984 (2011)
W. Rose, M. Creighton, D. Stewart, M. Sanwal, G. Trevithick, Can. J. Ophtalmol. 17, 61–66 (1972)
W. Williams, M. Cuvelier, C. Berset, Lebensm. Wiss. Technol. 28, 25–30 (1995)
L. Marcocci, L. Packer, M. Droy-Lefai, A. Sekaki, M. Albert, Methods Enzymol. 234, 462–475 (1994)
A.H. Kadhum, A.A. Al-Amiery, A.Y. Musa, A. Mohamad, Int. J. Mol. Sci. 12, 5747–5761 (2011)
C. Anuradha, J. Aukunuru, Trop. J. Pharm. Res. 9(1), 51–58 (2010)
J.A. Pople et al., Gaussian, 2004 Gaussian 03W (Revision C.01). Gaussian, Wallingford (2003)
W.J. Pietro, M.M. Francl, W.J. Hehre, D.J. Defrees, J.A. Pople, J.S. Binkley, J. Am. Chem. Soc. 104, 5039–5048 (1982)
K.D. Dobbs, W.J. Hehre, J. Comput. Chem. 8, 861–879 (1987)
A.D. Becke, Phys. Rev. A38, 3098–3100 (1988)
A.D. Becke, J. Chem. Phys. 98, 5648–5652 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B37, 785–789 (1988)
J.R. Soares, T. Dinis, P. Cunha, L. Almeida, Free Radic. Res. 26, 469–478 (1997)
D. Garratt, in The quantitative analysis of drugs, vol 3 (Chapman and Hall, Tokyo, 1964) 3 pp. 456–458
P.D. Duh, Y. Tu, G. Yen, Lebn. Wissen Technol. 32, 269 (1999)
Y. Chen, M. Wong, R. Rosen, C. Ho, J. Agric. Food Chem. 47, 2226–2228 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naama, J.H., Alwan, G.H., Obayes, H.R. et al. Curcuminoids as antioxidants and theoretical study of stability of curcumin isomers in gaseous state. Res Chem Intermed 39, 4047–4059 (2013). https://doi.org/10.1007/s11164-012-0921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0921-2