Abstract
An efficient chemoenzymatic process has been developed for preparation of 7-amino-3-[Z-2-(4-methylthiazol-5-yl)vinyl]-3-cephem-4-carboxylic acid, featuring removal of para-methoxybenzyl by trichloroacetic acid and cleavage of phenylacetyl E-isomer by immobilized penicillin acylase enzyme. The E-isomer of 7-amino-3-[Z-2-(4-methylthiazol-5-yl)vinyl]-3-cephem-4-carboxylic acid could be easily decreased to less than 0.2 % by salt formation. Importantly, trichloroacetic acid and immobilized penicillin acylase enzyme could be recovered and reused. The enzyme reaction could be run in a flow reactor. Only two crystallizations are involved as the purification procedure in the six-step sequence.



Similar content being viewed by others
Notes
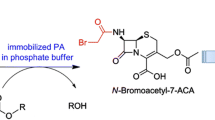
ATCA was isolated as white solid (m.p. 215–218 °C) in 90 % yield with 98 % purity containing 0.1 % E-isomer as determined by HPLC. The purity was analyzed by HPLC with commercial ATCA as reference [HPLC column: Hypersil BDS C18 diameter 4.6 × height 250 mm; mobile phase: 0.03 M phosphate buffer solution-acetonitrile (95:5 by volume); wavelength 254 nm]. ATCA analytical data: Purity 98 % (HPLC), m.p. 215–218 °C; [α]d + 185.3 (in 1 % NaHCO3, c 0.004). 1H NMR (DMSO-d6, 500 Hz) σ, 2.26 (s, 3H, CH3), 3.33 (d, 1H, CH, J = 18.5 Hz), 3.51 (d, 1H, CH, J = 18.5 Hz), 4.84 (d, 1H, CH, J = 5.0 Hz), 5.07 (d, 1H, CH, J = 5.0 Hz), 6.34 (d, 1H, CH, J = 12 Hz), 6.68 (d, 1H, CH, J = 12 Hz), 8.92 (s, 1H, CH).
References
K. Sakagami, K. Atsumi, A. Tamura, J. Antibiot. 38, 1047 (1990)
K. Sakagami, K. Atsumi, Y. Yamamoto, Chem. Pharm. Bull. 39, 2433 (1991)
K. Atsumi, K. Sakagami, Y. Yamamoto, EP 175610 (1986)
Y. Okada, M. Sakegawa, T. Watanabe, EP 1016665 (2000)
Y. Kumar, K. Singh, A. Prasad, WO 2005016936 (2005)
Y. Okada, M. Sukegawa, T. Watanabe, US patent 6288223 (2001)
Y. Kumar, M. Prasad, K. Singh, WO 2005100369 (2005)
U.P. Senthilkumar, P.K. Sahoo, A. Vempelli, WO 2007054777 (2007)
Y. Nishioka, M. Ito, Y. Kameyama, US patent 20080033166 (2008)
K. Prabhat, A. Vempali, S. Sundaravadivelan, WO 2005003134(2005)
Y. Nishioka, M. Ito, Y. Kameyama, EP 1752459 (2007)
Y. Nishioka, K. Sorajo, Y. Kanmeyama. US patent 20080064869 (2008)
Y. Kumar, M. Prasad, K. Singh, WO 2005100330 (2005)
K.M. Lee, P.E. Lim, Water Sci. Technol. 47, 41 (2003)
O.I. Kolodiazhnyi, Phosphorus ylides: chemistry and application in organic synthesis (Wiley-VCH Verlag, Weinheim, 1999), pp. 389–395
T.W. Greene, P.G.M. Wuts, Protective groups in organic synthesis (Wiley, New York, 1999), pp. 369–371
D.L. Boger, M. Hikada, B.M. Lewis, J. Org. Chem. 62, 1748 (1997)
J.F.J. Dippy, S.R.C. Hughes, A. Rozanski, J. Chem. Soc. (1959). doi:10.1039/JR9590002492
A. Liese, in Enzyme catalysis in organic synthesis, ed. by K. Drauz, H. Waldmann (Wiley-VCH Verlag, Weinheim, 2002), p. 14381441
Acknowledgments
The authors would like to thank the Hebei Research Center of Pharmaceutical and Chemical Engineering and the Pharmaceutical Molecular Chemistry Key Laboratory in Ministry of Technology. Support for Innovation Team Funding of Hebei University of Science and Technology is also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, C.H., Jia, C.G., Li, W. et al. A scalable chemoenzymatic process for 7-amino-3-[Z-2-(4-methylthiazol-5-yl)vinyl]-3-cephem-4-carboxylic acid (ATCA). Res Chem Intermed 39, 3911–3917 (2013). https://doi.org/10.1007/s11164-012-0907-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0907-0