Abstract
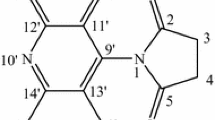
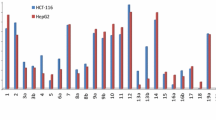
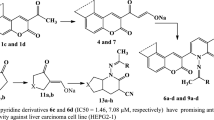
This study aims at the synthesis and evaluation of the chemotherapeutic activity of a number of 9-substituted tetrahydroacridine derivatives. The starting material, acridine hydrazide, could be prepared through the interaction between cyclohexanone and anthranilic acid, then chlorination of the product, then condensation of the last compound with hydrazine hydrate. The structures of the new compounds were established by IR, 1H NMR, MS spectra, and elemental analysis in certain cases. Antitumor activities as a trial to obtain more effective and less toxic agents were evaluated. The antitumor activity results indicated that the selected tetrahydroacridine derivatives showed antitumor activity against the liver cancer (HEPG2) tumor cell line tested, but with varying intensities in comparison to the known anticancer drugs, 5-fluorouracil and doxorubicin. It was found that compound VIb was the most active and induced a marked growth inhibition (0.694 μg/ml concentration) in a dose-dependent manner against liver cancer (HEPG2), while compound XVIIIa was second in regards to the growth inhibition activity (2.97 μg/ml concentration).





Similar content being viewed by others
References
L. Bouffier, M. Demeunynck, A. Milet, P. Dumy, J. Org. Chem. 69, 8144–8147 (2004)
J. Chiron, J.P. Galy, Synthesis (2004). doi:10.1055/s-2003-44379
H. Chen, Huaxuue Yanjiu Yu Yingyong (2000), 12, 164–168
S. Sivan, S. Tuchman, N. Lotan, Biosystems 70, 21–33 (2003)
S. Flock, C. Bailly, M.J. Waring, J.P. Henichart, P. Colsom, C. Houssier, J. Biomol. Struct. Dyn. 11, 881–886 (1994)
B.D. Gooch, P.A.J. Bontemps, Am. Chem. Soc. 126, 10610 (2004)
B. Stefanska, M.M. Bontemps Gracz, I. Antomini, S. Martelli, M. Arciemiuk, A. Piwkowska, D. Rogacka, E. Borowski, Bioorg. Med. Chem. 13, 1969–1975 (2005)
M. Demeunynck, Expert Opin. Ther. Pat. 14, 55–70 (2004)
A.M. Abdel-Halim, A.M. Tawfik, S.S. Ibrahim, A.M. El-Kazak, Indian J. Heterocycl. Chem. 3, 165–170 (1994)
Y.L. Chen, C.M. Lu, I.L. Chen, L.T. Tsao, J.P. Wang, J. Med. Chem. 45, 4689–4694 (2002)
Y.L. Chen, I.L. Chen, C.M. Lu, C.C. Treng, L.T. Tsao, J.P. Wang, Bioorg. Med. Chem. 11, 3921–3927 (2003)
J.S. Skotnicki, S.C. Gilman, US 851536. Chem. Abstr. 112, 118672 (1990)
S. Cai, Q.S. Li, R.T. Borchardt, K. Kuczera, R.L. Schowen, Bioorg. Med. Chem. 15, 7281–7287 (2007)
B.M. Rao, M.K. Sangaraju, P. Mahavan, M.L. devi, P.R. Kumar, K.B. Candrasekhar, Arpitha Ch., T.S. Balaji, J. Pharm. Biomed. Anal. 41, 1146–1151 (2006)
G. Hancu, A. Gaspar, A. Gyeresi, J. Biochem. Biophys. Methods 69, 251–259 (2007)
E. Bajetti, N. Zilembo, E. Bichisao, P. Pozzi, L. Toffolatti, Crit. Rev. Oncol. Hematol. 33, 137–142 (2000)
A. Demirbas, S. Ceylan, N. Demirbas, J. Heterocycl. Chem. 44, 1271–1280 (2007)
F.K. Guirgis, M.H. Ghanem, M.M. Abdel-Hay, Arzneim.-Forsch. 26, 435–440 (1976)
Y. Bashir, M. Kann, J.R. Stradling, Pulm. Pharmacol. 3, 151–154 (1990)
S.M. Cohen, E. Erturk, A.J. Von Esch, G.T. Bryan, J. Natl. Cancer Inst. 54, 841–849 (1975)
W.C. Patt, H.W. Hamilton, M.D. Taylor, M.J. Rryan, D.G. Taylor, C.J.C. Connolly, A.M. Doherty, S.R. Klutchko, I. Sircar, B.A. Steinbaugh, B.L. Batley, C.A. Painchaud, S.T. Rapundalo, B.M. Michniewicz, S.C.J. Olson, J. Med. Chem. 35, 2562 (1992)
J.C. Jaen, T.G. Heffiner, L.T. Meltzner, T.A. Pugsley, J. Med. Chem. 33, 311–317 (1990)
F.W. Bell, A.S. Cantrell, M. Hogberg, S.R. Jaskunas, N.G. Johansson, C.L. Jordan, M.D. Kinnick, P. Lind, J.M. Morin Jr, R. Noreen, B. Oberg, J.A. Palkowitz, C.A. Parrish, P. Pranc, C. Sahlberg, R.J. Ternasky, R.T. Vasileff, L. Vrang, S.J. West, H. Zhang, X.X. Zhou, J. Med. Chem. 38, 4929–4936 (1995)
J. Rudolph, H. Theis, R. Hanke, R. Endermann, L. Johannsen, F.U. Geschke, J. Med. Chem. 44, 619–626 (2001)
V. Cecchetti, G. Cruciani, E. Filipponi, A. Fravolini, O. Tabarrini, T. Xin, Bioorg. Med. Chem. 5, 1339–1344 (1997)
H. Kai, Y. Morioka, M. Tomida, T. Takahashi, M. Hattori, K. Hanasaki, K. Koike, H. Chiba, S. Shinohara, T. Kanemasa, Y. Iwamoto, K. Takahashi, Y. Yamaguchi, T. Baba, T. Yoshikawa, H. Takenaka, Bioorg. Med. Chem. Lett. 17, 3925–3929 (2007)
T.E. Ali, Phosphorus Sulfur Silicon Relat. Elem. 182, 1717–1726 (2007)
T.E. Ali, S.A. Abdel-Aziz, H.M. El-Shaaer, F.I. Hanafy, A.Z. El-Fauomy, Phosphorus Sulfur Silicon Relat. Elem. 183, 2139–2160 (2008)
T.E. Ali, M.A. Ibrahim, S.M. Abdel-Karim, Phosphorus Sulfur Silicon Relat. Elem. 184, 2358–2392 (2009)
H. Tiedtke, Chem. Ber. 42, 621–626 (1909)
R.A. Reed, J. Chem. Soc. (1944). doi:10.1039/JR9440000425
A. Albert, The Acridines, 2nd edn. (Arnold, London, 1966), p. 53
J.V. Braun, A. Heymons, G. Manz, Ber. Dtsch. Chem. Ges. 64, 227–235 (1931)
C. Fattorusso, G. Campiani, G. Kukreja et al., J. Med. Chem. 51(5), 1333–1343 (2008)
W.H. Perkin, W.G. Sedgwick, J. Chem. Soc. 125, 2437–2451 (1924)
H.K. Sen, U.P. Basu, J. Indian Chem. Soc. 6, 309–318 (1929)
U.P. Basu, S.T. Das Guptu, J. Indian Chem. Soc. 14, 458–461 (1937)
G.K. Hughes, F. Lions, J. Proc. Roy. Soc. New South Wales 71, 209 (1938)
J. Sairopoulos, N. El Batouti, A.M. Lamazouere, J. Heterocycl. Chem. 24, 907–912 (1987)
P. Skehan, et al., J. Natl. Cancer Inst. 82, 1107–1112 (1990)
L.M. Faddah, A.M. Soliman, Bull. Egypt Soc. Physiol. Sci. 14, 318–320 (1994)
A.M. Soliman, L.M. Faddah, Egypt J. Bilh. 16, 89–98 (1994)
Acknowledgments
The authors are grateful to the members of the Therapeutical Chemistry Department, Pharmaceutical and Drug Industries Division, National Research Centre, for helping to accomplish this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fathhalla, O.A., Mohamed, M.S., Farag, M.A. et al. Synthesis of certain tetrahydroacridine derivatives of anticipated medicinal value. Res Chem Intermed 39, 3487–3505 (2013). https://doi.org/10.1007/s11164-012-0857-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0857-6